Explore Any Narratives
Discover and contribute to detailed historical accounts and cultural stories. Share your knowledge and engage with enthusiasts worldwide.
The patient’s CT scan appeared unremarkable to the human eye. A subtle, diffuse shadow in the lower left lobe, easily dismissed as an artifact or minor inflammation. The AI saw something else. In February 2026, a diagnostic platform at Massachusetts General Hospital flagged that scan as high-risk, correlating its pixel patterns with a genomic database of early-stage lung adenocarcinoma profiles. A biopsy confirmed a stage I tumor, invisible to standard review. The patient started targeted therapy the following week.
This isn't science fiction. It is the operational reality of precision medicine in 2026, a field being fundamentally rewritten by artificial intelligence. The old paradigm—treating disease based on population averages—is collapsing. In its place, a new model is emerging: hyper-personalized, predictive, and powered by algorithms that find meaning in biological chaos. AI is the engine turning the promise of precision medicine from a conceptual ideal into a clinical standard, moving healthcare from a reactive stance to a proactive science.
Precision medicine’s core premise is simple: your biology is unique, so your healthcare should be too. For decades, the tools to realize this were blunt. We had genetics, but struggled to interpret the three billion base pairs of a genome. We had mountains of clinical data, trapped in unstructured physician notes. We had high-resolution imaging, but human fatigue limited its scrutiny. AI changes the equation not by introducing new data, but by giving us a new brain to comprehend it all.
This new brain thrives on integration. It fuses genomic sequences with proteomic profiles, metabolomic signatures with real-time streams from wearable biosensors. It reads a pathology slide not as a static image, but as a spatial map of cellular interactions. It parses a decade of electronic health records in milliseconds, finding hidden correlations between a childhood illness and a drug response thirty years later. The goal is no longer just diagnosis, but prediction—anticipating disease before symptoms manifest.
“We are moving from a medicine of ‘what do you have’ to a medicine of ‘what will you have, and how can we stop it,’” says Dr. Anya Sharma, Director of Computational Oncology at the University of Utah’s Department of Biomedical Informatics. “The AI models of 2026 don't just match drugs to mutations. They model disease as a dynamic, nonlinear process across multiple biological layers. They tell us about trajectory.”
The performance metrics are stark. Where traditional radiologist review for certain cancers might hover around 85% accuracy, AI-enhanced systems now consistently exceed 95%. The time from scan to finalized radiology report has collapsed from a day or two to between two and four hours. This speed and accuracy creates a cascade effect. Earlier detection means earlier intervention, which dramatically improves outcomes and reduces the burden of late-stage, costly treatments.
The poster child for precision medicine’s first wave was genomics. The problem was volume. Next-generation sequencing platforms like Illumina’s NovaSeq X can generate terabytes of raw genetic data per run. Interpreting that data was the bottleneck. AI, particularly deep learning models, has become the essential translator.
These models are trained on colossal, labeled datasets—like Illumina’s ambitious Billion Cell Atlas project launching this year—which map cellular states across diseases. They learn to identify patterns linking specific genetic variants to protein misfolding, or to predict how a tumor’s unique mutational signature will respond to a novel immunotherapy. This goes far beyond finding a known “cancer gene.” It’s about understanding the complex, emergent behavior of biological systems.
This capability is already moving from lab to clinic. The FDA’s approval of gene therapies for sickle cell disease was a landmark, but it’s just the start. AI-driven pharmacogenomics is now routinely used to match patients with the antidepressants, blood thinners, or chemotherapies least likely to cause adverse reactions and most likely to succeed, based on their individual enzyme profiles.
“The AI doesn't replace the genetic counselor or the oncologist,” notes Dr. Robert Chen, a lead bioinformatician at Illumina. “It augments them. It’s the difference between having a paper map of a continent and having a real-time, GPS-guided satellite image. You’re still driving the car, but you see every hill, every river, every potential roadblock long before you get there. The atlas we’re building with partners like AstraZeneca is about creating that complete map for human cellular function.”
If diagnostic AI is the expert eye, a new class of agentic AI is becoming the tireless orchestrator. These are not single-purpose algorithms, but autonomous systems that can plan, execute, and learn from sequences of actions within defined parameters. In healthcare, their impact is most profound in two areas: drug discovery and clinical workflow.
Developing a new drug is a famously slow, expensive, and failure-prone process. Agentic AI is compressing the timeline from years to months. These systems can generate thousands of novel molecular structures with desired properties, simulate their interactions with target proteins in silico, predict toxicity, and even design the early-stage clinical trial protocols to test them. They learn from each iteration, continuously refining the search for viable candidates.
Inside the hospital, AI agents are acting as clinical co-pilots. They monitor patient vitals streams from the Internet of Medical Things (IoMT)—smart beds, continuous glucose monitors, wearable ECG patches—and alert human staff only when patterns indicate genuine risk, like imminent sepsis or atrial fibrillation. They automate administrative drudgery: drafting clinical notes from doctor-patient conversations, prior authorization paperwork, and billing codes. This isn't about replacing nurses or scribes; it’s about freeing them from screens and letting them return to the bedside.
A surgeon in Barcelona now plans a complex spinal reconstruction not just with MRI scans, but with a 3D simulation generated by an AI that has analyzed hundreds of similar anatomies and outcomes. The robotic system, informed by this model, can then execute with sub-millimeter precision, minimizing tissue damage. The market for such AI-integrated surgical robotics is projected to explode from $5.16 billion in 2021 to nearly $21 billion by 2030. The result? Procedures that were once inpatient ordeals now see patients walking out the same day.
We stand at an inflection point. The technologies are moving from pilot projects to scaled implementation. The data infrastructures, from edge computing for private real-time analysis to federated learning systems that train algorithms across hospitals without sharing raw patient data, are falling into place. The question for 2026 is no longer “Can AI do this?” but “How fast can we integrate it ethically and equitably?” The diagnostic mind has arrived. The healthcare system is now learning how to listen to it.
Walk into any modern clinical genomics lab and the hum is not just from sequencing machines. It’s the sound of computation—servers parsing exabytes of biological data. The real transformation in precision medicine is happening here, in the engine room where AI models are being forged on datasets of unprecedented scale and complexity. This is where promise becomes protocol, and the market reflects the surge. According to Towards Healthcare, the AI in precision medicine sector is projected to explode from USD 4.32 billion in 2026 to USD 33.45 billion by 2035, a compound annual growth rate of 25.54%. This isn't speculative investment. It’s capital chasing proven, clinical impact.
The catalyst is projects like Illumina’s Billion Cell Atlas. Launched this year, it’s a moonshot effort to apply AI at a biological scale previously unimaginable. By mapping molecular pathways across a billion individual cells, the atlas provides the training data for a new generation of models. Pharmaceutical giants like AstraZeneca, Eli Lilly, and Merck are partners, not just observers. They’re using the platform to validate genetic drug targets with a speed that mocks traditional, plodding R&D timelines. The goal is hyperscale drug discovery: simulating interventions on a digital model of human biology before a single chemical is synthesized in a lab.
"The future of digital health is being shaped by the integration of diagnostics and AI to develop analytics to drive earlier diagnosis, predict risk of progression and indicate timely treatment interventions," observes a healthcare technology leader surveyed by Chief Healthcare Executive in 2026.
But does this computational brute force actually help the person in the oncology clinic today? The evidence says yes, and it’s delivered by platforms that bridge the infamous "last mile" between data and decision. First Ascent Biomedical’s Functional Precision Medicine platform represents a pragmatic, powerful approach. It doesn't just sequence a tumor; it tests live tumor cells against a panel of drugs, layers in genomic profiling data, and uses AI to rank treatment options. The result is a actionable report in days, not weeks. The company reports a staggering 83% patient benefit rate. That number should echo through every oncology department still relying on sequential, trial-and-error chemotherapy regimens.
Perhaps the most conceptually radical trend of 2026 is the emergence of the digital twin—a dynamic, AI-powered virtual replica of an individual patient. It’s not a static genomic profile. It’s a living simulation model that ingests continuous data streams: yesterday’s cortisol levels from a wearable, this morning’s metabolomic read from a smart toilet, last month’s cardiac MRI. Researchers can stress-test this twin with virtual treatments, predicting outcomes with a fidelity that turns medicine into a true engineering discipline. The potential is breathtaking, and the ethical questions are profound. Who owns the twin? Who is liable for its predictions?
Alongside the digital twin works the agentic AI, a tireless, logical orchestrator within the clinical workflow. These systems are moving beyond passive analysis to active assistance. They reduce the cognitive firehose faced by physicians.
"Agentic AI... will reduce time spent hunting for data, actively uncover overlooked insights and suggest evidence-based treatment pathways," says Craig Limoli, CEO of clinical AI company Wellsheet. The agent doesn't make the final call. It does the grueling reconnaissance, presenting the human commander with a synthesized intelligence briefing.
Adoption metrics from a 2026 global healthcare AI report sketch the portrait of a profession in mid-transformation. 59% of practitioners now use AI for diagnostics, primarily in imaging and predictive modeling. 57% leverage it for personalized treatment planning. More niche, but telling, is the 13% applying AI to precision nutrition for chronic conditions, and the 9% using it to draft routine patient communications. These numbers reveal a field pragmatically adopting tools that solve specific, painful problems—diagnostic uncertainty, administrative overload, chronic disease management.
For all the staggering statistics and silicon prowess, the clinic remains a human ecosystem. And here, the narrative gets messy. The integration of AI is creating a new kind of clinical divide. On one side are the adopters, clinicians who have learned to partner with algorithms, treating AI outputs as a powerful second opinion. On the other side are the skeptics, overwhelmed by yet another system to learn, distrustful of "black box" recommendations, and concerned about the erosion of clinical intuition.
This tension dominated sessions at conferences like the AI in Medicine 2026 summit. Debates rage around Evidence-Based AI Medicine (EB-AIM) versus traditional Evidence-Based Medicine. Regulatory bodies like the FDA are scrambling to create frameworks for continuously learning algorithms, not static devices. The core demand from physicians is for trustworthy AI—systems that don’t just provide an answer, but show their work, highlighting the imaging features or genomic markers that led to a conclusion.
"AI-supported precision medicine tailored to individual genetics, environment, and lifestyle will enable providers to predict Alzheimer’s or kidney disease, for example, years before symptoms appear," states a 2026 BCG report on AI agents. The promise is profound, but it hinges on a critical, unglamorous factor: data quality. Garbage in, gospel out. An AI trained on biased or incomplete datasets will perpetuate and even amplify healthcare disparities.
The industry’s response, for now, is a focus on high-impact, narrow pilots rather than wholesale transformation. As BCG analysts advise, success lies in executing a few transformative projects flawlessly, not dozens of superficial ones. We see this in the steady clinical adoption of next-generation sequencing for Minimal Residual Disease testing and Comprehensive Genomic Profiling in 2025. These are focused applications with clear clinical utility, and they are building the trust required for broader integration.
Is this cautious pace a failure of ambition or a mark of responsible maturity? Probably the latter. The specter of "alert fatigue" for clinicians—a constant barrage of AI-generated warnings—is a real danger. The technology must conform to human workflows, not the other way around. The most successful implementations are those that make the clinician’s job easier, not more complex. An AI that seamlessly prepopulates a clinical note from a patient conversation is adopted faster than one that demands a physician input 30 new data points for a risk score.
Much fanfare surrounds AI’s diagnostic prowess—finding the hidden tumor. But its most transformative role may be quieter: prediction. The real win isn't detecting stage I cancer; it’s identifying the patient who will develop it in five years and intervening now. This is the shift from pathology to probability.
Deep learning techniques, like those pioneered by researchers such as Zou and colleagues, are now efficiently interpreting full genomes not just for known disease variants, but for complex polygenic risk scores. These models can predict individual responses to common medications—antidepressants, statins, blood thinners—from genomic data alone, enabling pre-emptive genotyping so a doctor knows the optimal drug and dose at the point of prescription. This is pharmacogenomics made routine.
"Agentic AI... will reduce time spent hunting for data, actively uncover overlooked insights and suggest evidence-based treatment pathways," says Craig Limoli, CEO of clinical AI company Wellsheet. The agent doesn't make the final call. It does the grueling reconnaissance, presenting the human commander with a synthesized intelligence briefing.
Consider chronic kidney disease, a slow-motion crisis often detected only after significant, irreversible damage. Predictive models now fuse historical EHR data with real-time readings from connected devices, spotting the subtle trajectory toward dysfunction years before a standard test flags it. This is precision medicine at its most profound: not flashy intervention, but silent, continuous vigilance. It transforms healthcare from a repair service for broken biology into a maintenance system for human health.
Yet, a critical question persists. Does this data-intensive, AI-mediated future of medicine risk leaving behind those on the wrong side of the digital divide—the elderly, the poor, the rural populations without consistent broadband or access to genomic testing? The technology is democratizing in theory, but in practice, it could cement a two-tier system: predictive, preventative care for the data-rich, and reactive, conventional care for everyone else. The market may be growing at 25% a year, but who is that growth serving? The next great challenge for precision medicine isn't technical. It's equitable delivery. The algorithms are ready. Our healthcare systems, and our social contracts, are not.
The significance of AI in precision medicine transcends better diagnostics or faster drug discovery. It is forcing a fundamental redefinition of the human subject in healthcare. For centuries, the "patient" was a collection of presented symptoms and a limited history. The medical record was a sparse ledger of crises. The individual being treated was, in many ways, a biological black box. AI, fed by multi-omic data and continuous sensing, is prying that box open. The patient of 2026 is becoming a high-resolution, dynamic biological system that can be modeled, simulated, and understood in the context of their unique life. This isn't just a technical upgrade; it's a philosophical shift from treating disease to engineering health.
The cultural impact is already visible in the language of prevention. "What's your polygenic risk score for cardiovascular disease?" may soon be as common a question as "What's your cholesterol?" Personal health dashboards, powered by AI interpretations of wearable data, are shifting agency from the annual check-up to daily, individual monitoring. The historical legacy of this moment will be the end of the era of population-wide, one-size-fits-all medical guidelines. We are entering the age of the n-of-1 clinical trial, where each person's response to diet, drug, and environment constitutes a unique data point that refines their own care and contributes, anonymously, to the collective understanding of human biology.
"We are no longer just treating a disease label. We are treating a specific biological narrative that is unique to that individual at that point in time," says Dr. Elara Vance, a bioethicist specializing in AI at the Stanford Center for Biomedical Ethics. "The AI is the tool that finally allows us to read that narrative in its full complexity. The medical record becomes less of a legal document and more of a living biography of a body."
The industry upheaval is equally profound. The traditional, blockbuster drug model—find one drug for millions—is being supplanted by a pipeline of more targeted, often smaller-market therapies validated by AI against specific biomarker signatures. This changes the economics of pharma, favoring companies that can navigate biology with computational agility over those that rely on brute-force sales forces. Hospitals are transforming from fee-for-service repair shops into data-driven health management platforms. The power dynamics are shifting; tech companies with AI expertise and cloud infrastructure now sit at the table alongside venerable medical institutions, a partnership fraught with both potential and tension.
For all its brilliance, this new paradigm is built on foundations of sand. The criticisms are not minor quibbles; they are existential challenges that could derail the entire project if left unaddressed.
The most glaring issue is the black box problem. When a deep learning model recommends against a standard chemotherapy regimen, it can be impossible for a human oncologist to understand why. The model's reasoning is embedded in millions of weighted connections, not a logical flowchart. "Trust me" is not an acceptable basis for life-or-death decisions. Efforts in explainable AI are racing to catch up, but the field remains plagued by a fundamental trade-off: the most powerful models are often the least interpretable.
Then there is data bias, a poison in the well. AI models trained primarily on genomic and clinical data from populations of European descent perform poorly—and dangerously—for others. They can miss disease markers prevalent in other ancestries, recommend inappropriate drug doses, or simply fail to recognize pathology in non-white skin tones in imaging datasets. Without deliberate, costly curation of diverse datasets, AI-driven precision medicine threatens to precision-widen healthcare disparities, offering cutting-edge care only to those whose biology is well-represented in the digital atlas.
Access is the third fault line. The infrastructure required—whole-genome sequencing, continuous biosensors, high-performance computing for analysis—is expensive. The vision of a digital twin is a fantasy for the uninsured or the rural patient without reliable broadband. Will we create a medical caste system: the "data-rich" who receive predictive, preventative care, and the "data-poor" left with the crumbling legacy system of late-stage diagnosis? The technology democratizes in theory but stratifies in practice. Regulatory frameworks, particularly in the United States, are pathetically behind, still grappling with how to approve a static device, let alone an algorithm that learns and evolves every week.
Finally, there is the psychological burden of prediction. Knowing you have a 73% probability of developing early-onset dementia by age 60 is a form of knowledge that carries its own profound trauma. Our support systems for "patients-in-waiting" are non-existent. The AI can predict, but medicine has not yet developed the humanity to help people live with those predictions.
The upcoming AI in Medicine & Clinical Intelligence Congress in Berlin, scheduled for October 27-29, 2026, will have these criticisms at the top of its agenda, not as side discussions but as central themes. The focus is shifting from pure capability to responsible implementation.
Look for the release of the first major longitudinal study on AI-mediated polygenic risk score interventions in Q1 2027, led by a consortium at the Broad Institute. Its findings on patient outcomes and psychological impact will be a watershed. The prediction here is that by 2028, regulatory approval for any new clinical AI system will mandate not just proof of efficacy, but proof of interpretability and an audit for bias mitigation. The companies that survive will be those that built ethics into their architecture, not bolted it on as a public relations afterthought.
The patient in Barcelona whose stage I tumor was found by an algorithm glancing at a CT scan may never know the digital symphony that saved them. They only know the result: more time. That is the ultimate, undeniable power of this convergence. But the symphony is being composed on instruments we are still learning to play, from a score that is being written in real-time. The music is revolutionary. The responsibility to ensure it doesn't drown out the very humanity it seeks to serve is ours.
Your personal space to curate, organize, and share knowledge with the world.
Discover and contribute to detailed historical accounts and cultural stories. Share your knowledge and engage with enthusiasts worldwide.
Connect with others who share your interests. Create and participate in themed boards about any topic you have in mind.
Contribute your knowledge and insights. Create engaging content and participate in meaningful discussions across multiple languages.
Already have an account? Sign in here
AI revolutionizes medical physics, crafting precise radiation plans in minutes, transforming diagnostics, and reshaping ...
View Board
2026 marks a pivotal year for mRNA tech, with breakthroughs in cancer, HIV, microneedles, and AI-driven trials set to re...
View Board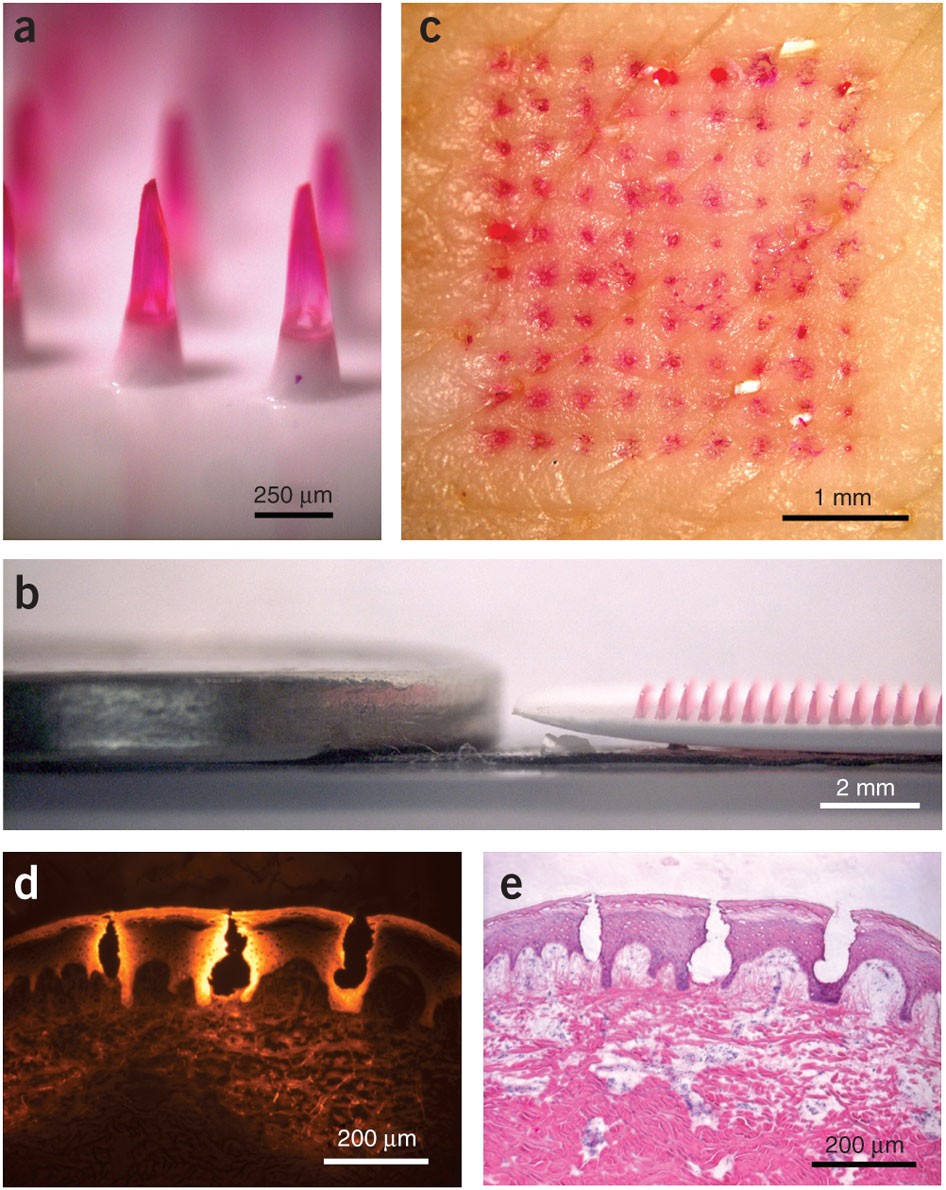
Microneedle patches deliver painless, effective vaccines via skin, revolutionizing global healthcare with self-administr...
View Board
Brain-computer interface breakthroughs create thought-controlled prosthetics, restoring motor control & realistic touch....
View Board
Major 2025 trials reveal no effective treatments for long COVID brain fog, forcing a shift from cognitive training to im...
View Board
Discover how AI is revolutionizing the fight against antibiotic-resistant superbugs. Learn about AI-driven drug discover...
View Board
MIT chemists synthesize verticillin A after 55 years, unlocking a potential weapon against fatal pediatric brain tumors ...
View Board
CES 2025 spotlighted AI's physical leap—robots, not jackets—revealing a stark divide between raw compute power and weara...
View Board
Discover Karl Landsteiner's groundbreaking work on blood groups (ABO & Rh), revolutionizing transfusions. Learn about hi...
View Board
Cancer research reaches new heights as ISS microgravity enables breakthroughs like FDA-approved pembrolizumab injections...
View Board
Tesla's Optimus Gen 3 humanoid robot now runs at 5.2 mph, autonomously navigates uneven terrain, and performs 3,000 task...
View Board
Hyundai's Atlas robot debuts at CES 2026, marking a shift from lab experiments to mass production, with 30,000 units ann...
View Board
The Architects of 2026: The Human Faces Behind Five Tech Revolutions On the morning of February 3, 2026, in a sprawling...
View Board
CES 2026 unveiled stair-climbing vacuums, mmWave presence sensors, and local AI robots that act, sense, and reshape smar...
View Board
Bis 2026 werden 80% der Unternehmen Physical AI nutzen – autonome Systeme, die nicht nur denken, sondern in Lagerhallen,...
View Board
Radiation-driven wolves in Chernobyl display rapid cancer-resistant evolution, a 30-year natural experiment revealing ge...
View Board
Steve Cutts and the Art of Satirical Social Commentary A man in a suit sits on a park bench, his face illuminated by th...
View Board
Tensor unveils Robocar at CES 2026: a Level 4 autonomous SUV with retractable wheel, 10 GPU clusters, and agentic AI, ta...
View Board
Die Reise von ELIZA zu proaktiven KI-Agenten: Wie aus reaktiven Chatbots autonome Akteure wurden, die Geschäftsprozesse ...
View BoardThe EU AI Act became law on August 1, 2024, banning high-risk AI like biometric surveillance, while the U.S. dismantled ...
View Board
Comments