Explore Any Narratives
Discover and contribute to detailed historical accounts and cultural stories. Share your knowledge and engage with enthusiasts worldwide.
Every year, a whisper of anxiety accompanies a routine mammogram. The wait for results can stretch for days, a limbo of uncertainty. For a radiologist, the pressure is immense: staring at hundreds of complex images daily, searching for the faintest shadow that could signal a life-altering disease. Now, a new kind of observer is joining them in the reading room—one that never blinks, never tires, and can process millions of historical scans in an instant. Artificial intelligence is moving from laboratory promise to clinical reality, fundamentally reshaping how we find cancer at its most beatable stage.
This isn't speculative futurism. Clinical-grade AI tools are already integrated into screening programs worldwide. In mammography, the frontline of breast cancer detection, multiple FDA-cleared AI products now assist radiologists. A large, prospective trial is currently underway in Sweden to measure their real-world impact. The evidence from earlier studies is compelling. These systems don't just match expert human performance; in some cases, they exceed it, demonstrating a consistent ability to reduce false negatives—the missed cancers that haunt every screening program.
The most profound shift may be from detection to prediction. A deep learning model called Mirai, developed at MIT and validated across multiple hospitals, can analyze a standard mammogram and predict a woman’s five-year risk of developing breast cancer. It looks beyond obvious tumors to subtle patterns in breast tissue density and texture, patterns invisible to the human eye. This isn't just about finding cancer today. It's about forecasting risk tomorrow, enabling a shift from one-size-fits-all screening to truly personalized, risk-stratified protocols.
“AI excels at finding the subtle patterns that are invisible or easily overlooked by humans. In screening, where you’re dealing with massive volumes of standardized data, that capability is transformative,” notes a 2024 review in Cancer Discovery.
The application extends far beyond breast tissue. In lung cancer screening with low-dose CT scans, deep learning models trained on vast datasets identify early, subtle nodules with precision that rivals seasoned radiologists. During colonoscopy, real-time AI systems highlight potential polyps on the monitor as the scope moves, increasing adenoma detection rates by as much as 50% in some studies. This metric is critical; finding and removing these precancerous growths is the definitive way to prevent colorectal cancer deaths.
Perhaps no example illustrates the practical, workflow benefits better than the transformation of cervical cancer screening. At the Medical University of South Carolina (MUSC) Hollings Cancer Center, an FDA-approved AI system called Genius Digital Diagnostics from Hologic is redefining the Pap smear. The system automates the entire slide-scanning process and uses AI to flag the most suspicious cells for a cytotechnologist’s review.
The impact on throughput is staggering. A cytotechnologist can now review approximately 200 AI-triaged slides in the same time it took to manually scrutinize 100. This effectively doubles laboratory capacity without adding staff, a crucial advantage amid chronic workforce shortages. The result is faster, more consistent results for patients and a system less prone to human fatigue.
“The AI doesn't replace our expertise; it augments it. It directs our attention to the areas that need it most, which means we’re not just faster, we’re more focused. That efficiency gets patients from an abnormal screen to a gynecologic oncologist much sooner,” explains a laboratory director at MUSC Health.
This acceleration is not merely administrative. A randomized trial published in 2024 demonstrated that using AI to triage suspicious mammograms reduced the median time from an abnormal screening exam to a biopsy by about 30%—shaving roughly 17 days off the diagnostic pathway. In that study, the AI-assisted pathway missed zero cancers. For a patient, those 17 days represent an eternity of worry. For a tumor, they represent potential progression.
While imaging dominates current screening, the next frontier is flowing through our veins. Liquid biopsy—the detection of cancer signals in blood—promises a less invasive path to early detection, especially for cancers like pancreatic or ovarian that lack standard screening tests. The challenge is monumental: finding a handful of cancerous cells or DNA fragments in a vast sea of normal biological material.
This is a problem engineered for AI. In October 2025, researchers at the University of Southern California announced a new AI tool named RED. The system can find rare circulating cancer cells in a blood sample in about ten minutes. Its key innovation is that it wasn't pre-programmed with specific cellular features to look for. Instead, it taught itself to recognize the aberrant patterns of cancer through deep learning. The team is already applying it to breast cancer, pancreatic cancer, and multiple myeloma, aiming to answer three fundamental questions: “Do I have cancer?”, “Is it gone or coming back?”, and “What is the best next treatment?”
The integration is happening across the entire cancer continuum. A 2025 oncology year-in-review highlighted that AI is now embedded in every stage of care, with the strongest evidence base materializing in screening, diagnosis, and risk prediction. The tools are moving from niche applications to mainstream clinical infrastructure. We are witnessing the early stages of a fundamental recalibration. The question is no longer if AI will be part of cancer screening, but how quickly and equitably its benefits can be scaled.
Clinical trials have moved beyond theoretical benchmarks. They are now measuring what happens when AI enters a real screening clinic. The results are shifting the conversation from "if it works" to "how well it works" and, more critically, "what changes." A sweeping meta-analysis of 31 studies, covering over two million screening exams, provides a definitive snapshot. When deployed as a second reader, AI generally maintained or even boosted sensitivity by up to nine percentage points while preserving specificity. The operational data reveals the true game-changer: triage systems. By using AI to filter out clearly normal exams, reading volumes plummeted by 40 to 90 percent. Radiologists could then focus their expertise on the ambiguous, high-risk cases where human judgment is irreplaceable.
"The most significant benefits emerge from triage configurations, which reduced reading volumes by 40-90% while maintaining non-inferior cancer detection," states the 2025 meta-analysis published in Radiology.
The prospective, randomized MASAI trial in Sweden delivered even more compelling evidence. This wasn't a retrospective look at old data; it was a live test. The AI-supported screening arm achieved a cancer detection rate of 6.4 per 1000 screenings, compared to 5.0 per 1000 in the standard care arm. That’s a statistically significant increase of 28 percent. Crucially, this higher detection did not come with a penalty of more false alarms or recalls. The AI helped find more real cancers without increasing patient anxiety or unnecessary procedures. This is the balanced outcome the field has been chasing for a decade.
The most startling breakthrough of 2025 came not from finding tumors, but from predicting how patients would respond to treatment. In August 2025, Caris Life Sciences published research demonstrating that an AI model could analyze standard hematoxylin and eosin (H&E) stained tissue slides—the most basic pathology image—and assess a critical immunotherapy biomarker called PD-L1. Traditionally, this requires a separate, specialized stain that can be inconsistent, especially near the 1% positivity threshold that often determines treatment eligibility.
The AI’s analysis had a direct, dramatic correlation with survival. For breast cancer patients treated with the immunotherapy drug pembrolizumab, those the AI scored as PD-L1 positive had a hazard ratio for overall survival of 0.511. This means their risk of death was nearly halved compared to AI-negative patients. The traditional immunohistochemistry method, by contrast, yielded a non-significant hazard ratio of 0.882. The AI’s read was more predictive of who would actually benefit from a powerful, expensive, and potentially toxic treatment.
"Traditional PD-L1 testing can undercall positive cases, especially near the 1% threshold. Our AI model enhances predictive accuracy and exhibits superior prognostic precision compared to current biomarker assessments." — Dr. Matthew Oberley, SVP and Chief Clinical Officer at Caris Life Sciences
This changes the fundamental role of AI in oncology. It’s no longer just a detection aid; it’s a prognostic and therapeutic guide. The algorithm is seeing biological subtleties in tissue architecture and cellular arrangement that are completely invisible to a pathologist’s eye, patterns that directly correlate with how a tumor will behave. The implications are enormous for treatment selection across cancer types.
Adoption is accelerating because the technology solves a practical crisis: unsustainable workload. Radiologists and pathologists are drowning in data. AI offers a lifeline. At the Summit Cancer Center, the integration is holistic. Dr. Arving Chaudhry describes an environment where AI is woven into the entire fabric of care, from diagnostics through treatment planning and data abstraction.
"The next wave of technology is all about AI. It is becoming part of every stage of cancer care, and that's very exciting." — Dr. Arving Chaudhry, director of Summit Cancer Center
The efficiency gains are quantifiable and transformative. Consider the grueling, expensive process of abstracting clinical data from patient records for research or registries. Dr. Hoifung Poon of Microsoft Research lays out the staggering scale of the old way versus the new. With twenty million new global cancer patients annually, manual abstraction requires hundreds of dollars and hours of human time per case. A GPT-4 powered system accomplishes the same task in seconds for cents. This liberates human expertise for higher-order tasks and dramatically lowers the barrier for large-scale, real-world evidence generation.
But does this flood of automation create distance between doctor and patient? A valid concern, but the current implementation suggests the opposite. By handling the volumetric drudgery—the initial scan of a thousand normal mammograms, the sorting of Pap smear slides—AI gives specialists more cognitive bandwidth for the complex cases that need them. The technology at the University of Arkansas, focused on explaining how its AI arrives at a conclusion for chest scans, points to the next necessary step: interpretability. The goal isn't a black box that barks orders; it's a collaborative tool that highlights areas of concern and explains its reasoning, allowing the radiologist to make a faster, more informed final call.
Hologic’s data on mammography underscores this collaborative power. In a study of 7,500 screening exams, their AI flagged approximately one-third of breast cancer cases that radiologists had initially interpreted as negative. This isn’t an indictment of human skill. It’s a demonstration of potent synergy. The AI acts as a relentless, consistent second pair of eyes, catching the subtle signs of disease that can slip past anyone on a long, taxing day. The result isn't replacement; it's reinforcement.
For all the momentum, the path forward is not a smooth, paved highway. It is littered with significant, unresolved obstacles. The most dangerous is bias. An AI model is only as good, and as fair, as the data it was trained on. Most large, annotated datasets come from major academic medical centers in North America and Europe, featuring predominantly populations of specific ethnic and socioeconomic backgrounds. What happens when an algorithm trained on this homogeneous data encounters a screening mammogram from a rural clinic in Southeast Asia, or from a patient of West African descent with different breast density patterns? The performance can degrade, sometimes silently. The very tool meant to democratize care could instead exacerbate existing health disparities, systematically failing certain groups.
"Definitive evidence on safety, especially interval cancer outcomes, remains essential before considering AI as a stand-alone reader," cautions the Radiology meta-analysis, highlighting that the long-term, population-level data we have for human screening programs simply doesn't exist yet for AI.
Then there is the specter of overdiagnosis. Increased sensitivity is a double-edged sword. Finding more cancers sounds unequivocally positive, but what if AI is exquisitely talented at finding tiny, indolent tumors that would never have threatened a patient’s life? We already grapple with this in prostate cancer (PSA testing) and some breast cancers. AI could supercharge this problem, leading to a surge in biopsies, surgeries, and radiotherapy for conditions that didn't require treatment. The psychological and physical toll of this "overdiagnosis cascade" could offset the benefits of finding lethal cancers earlier. The field lacks the longitudinal studies to know where the new sensitivity-optimized AI tools will land on this critical spectrum.
Integration remains a messy, practical headache. These are not plug-and-play devices. They require seamless integration into legacy hospital IT systems, PACS networks, and clinician workflows. They demand new protocols, new training, and new liability frameworks. Who is responsible if an AI misses a cancer it was supposed to flag? The hospital, the radiologist who overruled it, or the software company? Regulatory bodies like the FDA are playing catch-up, struggling to adapt approval pathways designed for static medical devices to algorithms that learn and evolve.
And beneath it all lies a foundational question: are we building systems that help doctors, or systems that seek to replace them? The stated goal is augmentation, but the economic pressures of healthcare are relentless. A hospital administrator looking at a 90% reduction in screening mammogram reading time might see a path to drastic cost-cutting. This tension between clinical benefit and financial incentive will define the rollout of this technology. The risk is a two-tiered system: AI-augmented excellence for some, and fully automated, minimally-supervised algorithmic screening for the underserved. That would be a tragic perversion of the technology's promise. The optimism is warranted, but it must be tempered with rigorous, independent scrutiny and a steadfast commitment to equity. The algorithm itself is amoral; its application will determine its legacy.
The significance of AI-powered screening transcends the immediate goal of finding tumors earlier. It represents a fundamental reorientation of the entire medical model from reactive sickness care to proactive health management. For decades, screening has been a blunt, population-wide instrument: everyone of a certain age gets the same test at the same interval. AI, particularly through tools like the Mirai risk predictor, shatters that paradigm. It enables a shift to dynamic, individualized surveillance where screening frequency and modality are tailored to a person’s continuously updated risk profile. This isn't just incremental improvement; it's the foundation for precision prevention.
The cultural impact is subtler but just as profound. It changes the relationship between patient and data. Your mammogram is no longer a snapshot judged solely for immediate abnormalities. It becomes a data point in a lifelong, personalized risk trajectory. It empowers a more informed dialogue between patient and physician, moving from a binary "clear" or "suspicious" result to a nuanced discussion about probability and prevention strategies. The industry impact is already catalyzing a new ecosystem. Traditional medical imaging companies are now AI software firms. Pathology labs are becoming computational biology hubs. A new category of clinical professional—the AI validation specialist—is emerging.
"AI is touching every aspect of cancer care. It is becoming part of every stage, and that's very exciting because it allows us to move from a one-size-fits-all approach to truly personalized cancer management." — Dr. Arving Chaudhry, director of Summit Cancer Center
The legacy of this moment will be measured in timelines. The 30% reduction in time-to-biopsy demonstrated in trials translates to weeks of agonizing uncertainty erased from a patient's life. The doubling of Pap smear throughput at MUSC translates to thousands of women receiving potentially life-saving results sooner. These are not abstract metrics; they are compounding dividends of human benefit, reducing the systemic friction that allows cancers to progress.
For all its promise, the critical perspective demands we confront the unresolved equation. The ethical and practical challenges are not mere footnotes; they are central to the technology's ultimate success or failure. The "black box" problem persists. While explainable AI is a growing field, many of the most powerful deep learning models operate in ways even their creators cannot fully interpret. When an AI flags a mammogram as high-risk or a tissue sample as PD-L1 positive, can we truly explain why? This creates a profound medico-legal and philosophical dilemma. Can a physician, let alone a patient, trust a diagnosis without understanding its genesis?
Access is the other looming fault line. The initial deployment of these expensive, compute-intensive systems will naturally flow to well-resourced institutions in wealthy nations. This threatens to create a devastating "AI divide" in global health. The very tools that could reduce disparities might instead cement them, offering superior early detection to the affluent while the underserved rely on older, human-only systems. The business model itself is a concern. Will AI screening tools be licensed as proprietary software, creating recurring costs that strain public health budgets? Or will they evolve as open-source platforms, validated and adapted by the global community?
Finally, there is the question of clinical over-reliance. The danger isn't that AI will replace doctors overnight, but that it will subtly deskill them. If a generation of radiologists is trained to rely on AI triage, will they lose the pattern recognition skills to spot the truly bizarre, the never-before-seen presentation that falls outside the algorithm's training data? The technology must be a scaffold for expertise, not a crutch that allows it to atrophy.
The forward look is specific and grounded in ongoing research. The Swedish MASAI trial will continue to yield long-term outcome data through 2026, providing the first robust evidence on whether AI-assisted screening actually reduces advanced cancer incidence and mortality at a population level. In the United States, watch for the expected FDA decision on the first autonomous AI reading system for a specific screening modality, likely in mammography, which will trigger fierce debate about the role of human oversight.
The most concrete development will be the move from single-modality to multi-modal AI integration. Research in 2026 will aggressively combine data streams: imaging, liquid biopsy results from tools like the RED platform, genomic risk scores, and even lifestyle data from wearables. The goal is a unified, AI-synthesized "health risk dashboard" that provides a holistic early-warning system. Clinical trials for these integrated platforms are already in the planning stages at major cancer centers like MD Anderson and Memorial Sloan Kettering, with pilot studies expected to launch by the second quarter of 2026.
The prediction, based on the current trajectory, is that within three years, AI will become the unremarkable, standard first reader for high-volume screening exams like mammograms and low-dose CT lung scans in most advanced health systems. Its role will be tacit, like spell-check in a word processor—always on, mostly invisible, correcting subtle errors and directing attention. The human expert will remain firmly in the loop, but their role will elevate from primary scanner to final arbiter and interpreter of complex cases.
That radiologist, in a reading room perhaps five years from now, will face a different kind of quiet. The crushing volume of normal scans will have been filtered into a digital repository, marked "AI-reviewed, no findings." Their monitor will display only the curated, complex cases where the algorithm expressed uncertainty or spotted something subtle. Their expertise, honed and unburdened, will be focused precisely where it is most needed. The anxiety-laden wait for patients will shrink from weeks to days. And the whisper of a tumor will be heard not in the silence of a missed diagnosis, but in the efficient, collaborative hum of human and machine intelligence working in concert. The question is no longer if we can find cancer earlier, but whether we have the wisdom to build a system that ensures everyone can.
Your personal space to curate, organize, and share knowledge with the world.
Discover and contribute to detailed historical accounts and cultural stories. Share your knowledge and engage with enthusiasts worldwide.
Connect with others who share your interests. Create and participate in themed boards about any topic you have in mind.
Contribute your knowledge and insights. Create engaging content and participate in meaningful discussions across multiple languages.
Already have an account? Sign in here

ZDoggMD transforms medical burnout into viral rap satire, exposing systemic flaws while building direct‑care models that...
View Board
Cancer research reaches new heights as ISS microgravity enables breakthroughs like FDA-approved pembrolizumab injections...
View Board
MIT chemists synthesize verticillin A after 55 years, unlocking a potential weapon against fatal pediatric brain tumors ...
View Board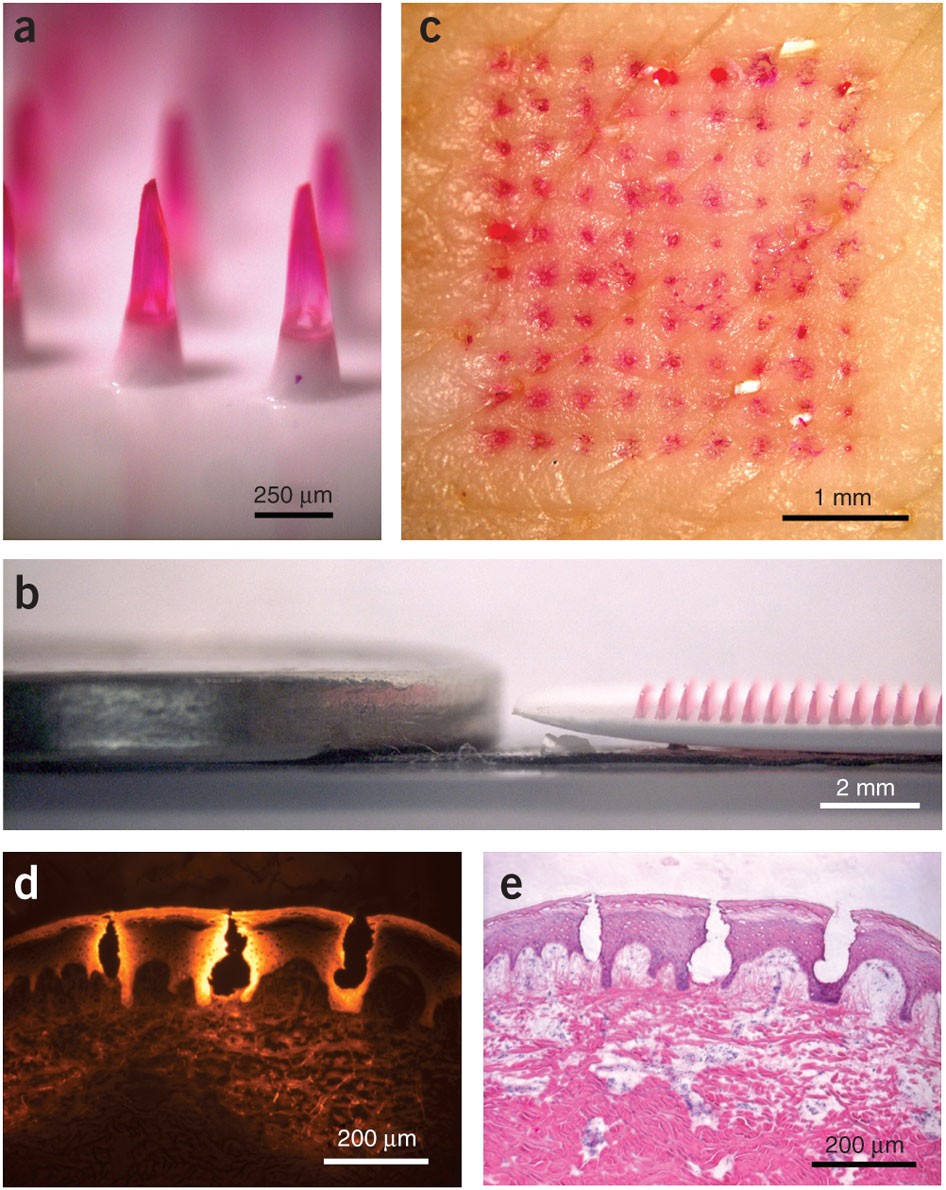
Microneedle patches deliver painless, effective vaccines via skin, revolutionizing global healthcare with self-administr...
View Board
Brain-computer interface breakthroughs create thought-controlled prosthetics, restoring motor control & realistic touch....
View Board
Discover how AI is revolutionizing the fight against antibiotic-resistant superbugs. Learn about AI-driven drug discover...
View Board
Journalist details Walter Freeman's 1946 ice-pick lobotomy, its 10-minute office speed, 2,500 operations, and the enduri...
View Board
AI revolutionizes medical physics, crafting precise radiation plans in minutes, transforming diagnostics, and reshaping ...
View Board
Entdecken Sie das Leben von Robin Warren, dem medizinischen Pionier, der mit der Entdeckung von Helicobacter pylori die ...
View Board
Jonathan Tomines: The Toe Bro's Unlikely Path to Fame The screen shows a human foot, its big toe swollen and angry. A s...
View Board
Pancreatic cancer's sugar-coated shield uncovered: Researchers reveal how tumors exploit sialic acid to deceive immune c...
View Board
Discover Karl Landsteiner's groundbreaking work on blood groups (ABO & Rh), revolutionizing transfusions. Learn about hi...
View Board
Discover how Sir Ronald Ross revolutionized malaria understanding! Learn about his groundbreaking discovery of mosquito ...
View Board
Discover Tu Youyou's groundbreaking discovery of artemisinin, a life-saving antimalarial drug. Learn about her journey, ...
View Board
Major 2025 trials reveal no effective treatments for long COVID brain fog, forcing a shift from cognitive training to im...
View Board
Google LiteRT redefine IA em dispositivos pequenos, com execução local, baixa latência e eficiência energética em smartp...
View Board
Julian Baumgartner: The Alchemist of Art's Past, Shaping Its Present The hushed reverence of the museum gallery is shat...
View Board
KI findet 25 neue Magnete für die nächste Generation der Elektromobilität Der Motor eines Elektroautos surrt fast unhör...
View Board
2026 marks a pivotal year for mRNA tech, with breakthroughs in cancer, HIV, microneedles, and AI-driven trials set to re...
View Board
MIT’s 2026 breakthroughs reveal a world reshaped by AI hearts, gene-edited embryos, and nuclear-powered data centers, wh...
View Board
Comments