Félix d'Herelle: The Discoverer of Bacteriophages
Félix Édouard Victor d'Herelle (1873-1954) was a French-Canadian microbiologist renowned for his pioneering work in bacteriophage virology. His revolutionary discoveries led to the development of a new field, phage therapy, and significantly advanced our understanding of viruses that infect bacteria. This article delves into d'Herelle's life, his scientific journey, and the impact of his work.
Early Life and Education
Édouard Félix d'Herelle was born on November 14, 1873, in Saint-Hyacinthe, Quebec. His early life was marked by a keen interest in science. He completed his primary and secondary education in his hometown, where he laid the foundation for his future scientific endeavors. In 1895, d'Herelle pursued a medical degree at the Faculty of Medicine in Montreal, which provided him with the necessary tools and knowledge to embark on a career in microbiology.
Early Career and Scientific Breakthrough
Upon graduating in 1900, d'Herelle started working as a microbiologist at the National Institute for Agriculture in France. His journey as a scientist began in earnest in 1908 when he discovered a phenomenon that would later be known as bacteriophages. This discovery occurred when he was studying an outbreak of dysentery in Egypt. He observed that when he added cultures of the pathogen causing the dysentery to an infected culture medium, the infection was halted. The substance responsible for this effect was later identified as a virus that specifically targeted and destroyed bacterial cells, marking the first observed instance of a virus infecting a bacterium.
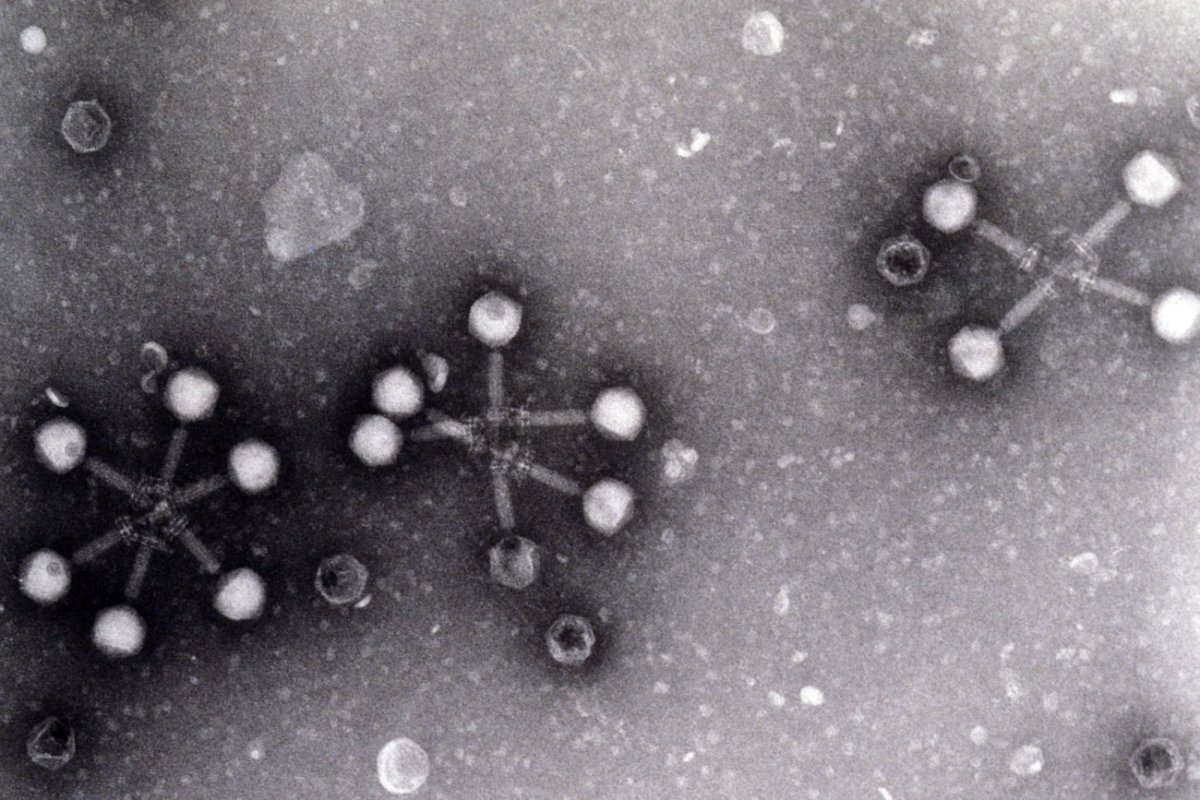
The Discovery of Bacteriophages
During his research, d'Herelle hypothesized that the substance he discovered was a virus, which he termed a "bacteriophage." The term "bacteriophage" is derived from the Greek "phagein," meaning "to devour." D'Herelle's discovery was a groundbreaking moment in microbiology, as it was the first time a virus was identified and understood to have such specific properties. This event laid the foundation for the emerging field of molecular biology and laid the groundwork for future studies into virology.
Developing Phage Therapy
Recognizing the potential of these bacteriophages to combat bacterial pathogens, d'Herelle envisioned the creation of phage therapy. This method of using bacteriophages to fight bacterial infections offers a non-toxic and non-specific approach to treatment, which is a significant advantage over antibiotics. However, d'Herelle's ideas were met with skepticism in the medical community, particularly in France and the United States, which were already well-versed in the use of antibiotics. Despite this, his work gained traction in the Soviet Union, where phage therapy was initially given more attention and funding.
Expanding Knowledge and Influence
In 1915, d'Herelle emigrated to the United States to expand his work on phage therapy. He joined the International Phageological Society, which he co-founded, and became a pivotal figure in promoting the use of phages in medical research and clinical practice. His travels and collaboration with scientists around the world furthered the understanding of bacteriophages and spurred the development of phage therapy as a therapeutic option.
Challenges and Legacy
While d'Herelle's research was pioneering, it faced numerous challenges. Antibiotic resistance and the reluctance of the scientific community to accept his findings were significant obstacles. The 1930s saw a decline in phage research due to advancements in antibiotic development, but the revival of interest in phage therapy began in the 1960s with the discovery of viruses that could be engineered to target specific bacterial pathogens, leading to a new wave of research and treatment options.
Awards and Recognition
For his groundbreaking work, d'Herelle received numerous accolades and honors. In 1928, he was awarded the Pasteur Medal by the Pasteur Institute in France, recognizing his significant contributions to the field of microbiology. His work continued to be recognized with the Lomonosov Gold Medal in 1948 from the Soviet Union, reflecting the international acknowledgment of his scientific legacy.
Personal Life and Legacy
Despite the challenges, d'Herelle remained a dedicated scientist and educator. He authored numerous papers and books, including "Maladies Bactériologiques," which detailed his findings on bacteriophages. His personal life was marked by both success and tragedy; he married Marguerite Héroux in 1905, and they had three children. Tragically, his eldest daughter died in 1916, which deeply affected d'Herelle's later life. He passed away in 1954 in Paris, leaving behind a legacy that continues to inspire researchers in microbiology and virology.
In conclusion, Félix d'Herelle's contributions to the field of microbiology and virology were monumental. His discovery of bacteriophages opened new avenues for research and therapy, leaving a lasting impact on the scientific community. His work continues to influence and inspire scientists, and the resurgence of interest in phage therapy underscores the enduring significance of his pioneering research.
The Development of Phage Technology
Throughout the early 20th century, d'Herelle continued to refine his methods for producing and using bacteriophages in therapeutic settings. His work laid the groundwork for the development of phage technology, which involves the application of bacteriophages as a form of biological control for bacterial infections. This technology has since evolved to include the use of phages for various purposes, such as biocontrol in agriculture and biosecurity in food safety.
Phage Research in the United States
Following his move to the United States, d'Herelle established the Eliava Institute of Microbiology in Tbilisi, Georgia, in 1923. This institute became the nerve center for the study and application of phage therapy. The institute was named after Ilia Ivanovich Eliava, a pioneering Georgian microbiologist who collaborated with d'Herelle. Here, d'Herelle’s team conducted extensive research and developed practical applications for phage therapy. They demonstrated that phages could be used to treat a wide range of infections, from skin abscesses to more severe illnesses like cholera and typhoid fever.
The success of phage therapy at the Eliava Institute was particularly notable during the flu pandemic of 1918, where it was used to treat tens of thousands of patients. Although the full extent of its impact is debated, the institute’s work gained worldwide attention and recognition. In 1926, d'Herelle published his seminal book, "Sur la thérapie des maladies bactériologiques par les phages," which detailed his findings and the potential of phage therapy.
Phage Therapy and Medical Practice
Despite the initial promise, the widespread adoption of phage therapy faced numerous challenges. The emergence and widespread acceptance of antibiotics in the 1940s provided a more immediate and effective solution to bacterial infections, leading to a decline in research and funding for phage therapy. However, the limitations of antibiotics, such as resistance development, led to a resurgence of interest in phage therapy in the latter half of the 20th century.
One of the most significant challenges in the medical practice of phage therapy was the variability of phage strains. Each strain targets a specific bacterium, and the development of a cocktail of phages for a particular infection required a high degree of specificity and customization. This complexity made the therapy difficult to standardize and implement on a broader scale.
Advancements in Phage Research
As scientific understanding of bacteriophages and their mechanisms of action evolved, new research approaches emerged. In the 1960s, the discovery of restriction enzymes by Ham Smith and his colleagues at the Cold Spring Harbor Laboratory opened up new avenues for manipulating and engineering phages. This breakthrough allowed scientists to create recombinant phages with enhanced properties, such as improved stability and specificity. These advancements laid the foundation for the modern era of phage therapy.
Advances in molecular biology also played a crucial role in the revival of interest in phage therapy. Techniques such as electroporation and transformation were developed, enabling the introduction of genetic material into phages. This capability was further enhanced by the CRISPR-Cas9 system, which allowed for precise gene editing in phages, tailoring them to target specific bacterial genomes. These techniques have significantly improved the design and application of phage therapies.
Phage Therapy Today
Today, phage therapy is gaining renewed interest in both research and clinical settings. Numerous studies and clinical trials are underway to evaluate the efficacy and safety of phage therapy for various bacterial infections. The success of phage therapy in combating antibiotic-resistant bacteria is especially promising. For example, in 2018, the first-ever FDA-approved phage therapy was approved for use in the treatment of a patient with antibiotic-resistant infections caused by Pseudomonas aeruginosa.
However, challenges remain. One of the primary hurdles is the high cost of developing and producing phage cocktails for specific pathogens. Additionally, the lack of standardization and regulatory approval in many countries presents a significant barrier to widespread adoption. Nonetheless, the potential of phage therapy is undeniable, and ongoing research aims to overcome these challenges.
Future Prospects and Innovations
The future of phage therapy holds significant promise. Ongoing research is exploring the development of phages with broader and more specific targeting capabilities. This includes the use of multi-target phage cocktails and the creation of phage-display libraries to screen for optimal phage-antibiotic combinations. These innovations are expected to enhance the efficacy and versatility of phage therapy.
Furthermore, the integration of phage therapy with other treatment modalities, such as antimicrobial peptides and antibodies, is being investigated. This multi-faceted approach could provide a more comprehensive and effective solution for bacterial infections. Additionally, advances in artificial intelligence and machine learning are being used to optimize phage selection and delivery systems, promising even more targeted and efficient therapies.
Conclusion
Although Félix d'Herelle's work on bacteriophages was initially met with skepticism, his legacy has endured. The development and ongoing research in phage therapy continue to build on his foundational discoveries. As the challenges of bacterial resistance persist, phage therapy emerges as a promising alternative and complementary approach. The future of this field looks bright, with continued advancements promising to unlock new horizons in the treatment of bacterial infections.
Global Impact and Recognition
Félix d'Herelle's work extended beyond the laboratory and into the global public health arena. His discoveries and the subsequent development of phage therapy had a profound impact on medical practice and public health policy in various regions. The Russian and Georgian governments provided substantial support for the development and application of phage therapy, leading to its widespread use in these countries during the early 20th century.
The success of phage therapy in treating bacterial infections during the 1918 flu pandemic, as well as other outbreaks, provided concrete evidence of its effectiveness. This evidence was instrumental in gaining international recognition and support for phage therapy. In 1926, d'Herelle traveled to the United States to present his findings at the American Medical Association and the American Public Health Association. His lectures and demonstrations of phage therapy were met with considerable interest, although the broader medical community remained skeptical.
The Eliava Institute in Tbilisi continued to be a hub for phage research and treatment until the fall of the Soviet Union. After its closure, many of the institute's researchers emigrated to the United States and other countries, bringing their knowledge and expertise with them. This diaspora of phage researchers helped to rekindle interest in phage therapy in the West and laid the groundwork for future advancements.
Challenges and Modern Revival
Despite the initial excitement, the widespread adoption of phage therapy faced numerous challenges. The lack of standardized protocols, the complexity of producing phage cocktails, and the limited regulatory approvals in many countries were significant barriers. However, the resurgence of antibiotic resistance in the late 20th and early 21st centuries has led to a renewed interest in phage therapy.
Modern scientific advances have helped to overcome some of these challenges. Improved methods for phage production and delivery, as well as advances in genetic engineering, have made phage therapy more viable. The CRISPR-Cas9 system, for example, allows for precise editing of phage genomes, enhancing their specificity and effectiveness. Additionally, the development of phage libraries has made it possible to screen for phages with specific targets, significantly increasing the efficiency of the therapy.
Phage Therapy in Clinical Practice
In recent years, there has been a growing body of evidence supporting the clinical effectiveness of phage therapy. Numerous studies have shown that phage therapy can be effective against antibiotic-resistant bacteria, such as Pseudomonas auruginosa, Acinetobacter baumannii, and Enterobacteriaceae. Clinical trials are currently ongoing to evaluate the safety and efficacy of phage therapy for a range of bacterial infections, including those that are difficult to treat with conventional antibiotics.
One notable example is the approval of the first FDA-regulated phage therapy. In 2022, the FDA approved a phage therapy for the treatment of recurrent skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus (MRSA). This approval marks a significant milestone in the integration of phage therapy into mainstream medical practice.
Future Directions and Research
The future of phage therapy is promising, with ongoing research aimed at improving its efficacy and safety. Key areas of focus include the development of more specific and targeted phage cocktails, the optimization of delivery systems, and the integration of phage therapy with other antimicrobial strategies. For example, the combination of phage therapy with antibiotics or other antimicrobial agents holds great potential for treating multidrug-resistant infections more effectively.
Moreover, advances in artificial intelligence and machine learning are being used to optimize phage selection and delivery. These technologies can help identify the most effective phage cocktails for specific bacterial strains, thereby increasing the likelihood of successful treatment. Additionally, the use of phages in biocontrol and agriculture, particularly in reducing the use of synthetic pesticides, is an emerging area of research with significant environmental benefits.
Conclusion and Legacy
Félix d'Herelle's legacy extends far beyond his initial discovery of bacteriophages. His pioneering work and the development of phage therapy have opened new frontiers in the fight against bacterial infections. While challenges remain, the potential of phage therapy is undeniable. As research continues to advance, the future of phage therapy holds significant promise for addressing the ever-evolving threat of antibiotic resistance.
The story of Félix d'Herelle and his pioneering work serves as a testament to the transformative power of scientific discovery and the enduring impact of visionary thinking in the face of skepticism. His legacy continues to inspire and guide researchers and medical practitioners, ensuring that phage therapy remains a vital tool in the fight against bacterial infections.














Comments