Francisco Mojica: The Pioneer Behind CRISPR’s Revolutionary Discovery
Introduction
When discussing the groundbreaking gene-editing technology CRISPR-Cas9, names like Jennifer Doudna and Emmanuelle Charpentier often dominate the headlines. However, the foundation of this revolutionary tool was laid by an unassuming Spanish microbiologist, Francisco J. Martínez Mojica. Mojica’s decades-long research into the repetitive DNA sequences in archaea and bacteria led to the discovery of CRISPR, a biological mechanism that has since transformed genetic engineering, medicine, and biotechnology. This article explores Mojica’s journey, his pivotal discoveries, and the profound impact of his work on modern science.
Early Life and Academic Background
Francisco Juan Martínez Mojica was born in 1963 in Elche, a small town in southeastern Spain. From an early age, he displayed a keen interest in biology, fascinated by the microscopic world and the complexity of living organisms. He pursued his passion by enrolling at the University of Alicante, where he earned a degree in biology in 1986. His academic journey continued with a Ph.D. in microbiology, focusing on halophilic microorganisms—organisms that thrive in extremely salty environments—such as those found in the salt marshes of Santa Pola, near his hometown.
Mojica’s doctoral research at the University of Alicante set the stage for his later discoveries. Working under the mentorship of Professor Francisco Rodríguez-Valera, he delved into the genetic adaptations of extremophiles, microorganisms that survive in harsh environments. Little did he know that his work would eventually lead to one of the most significant biological breakthroughs of the 21st century.
The Discovery of CRISPR: A Scientific Milestone
In the early 1990s, while sequencing the DNA of Haloferax mediterranei, a salt-loving archaeon, Mojica noticed something unusual. The genome contained peculiar repetitive DNA sequences—short, palindromic segments interspersed with unique spacer regions. These sequences were unlike anything documented before, and their function was entirely unknown. Intrigued, Mojica dedicated the next decade to understanding their role.
Initial research suggested that these sequences might be involved in genome organization or DNA repair, but Mojica suspected something more profound. By analyzing microbial genomes from various environments, he found similar repeating patterns in other archaea and bacteria. In 2000, Mojica coined the term "CRISPR" (Clustered Regularly Interspaced Short Palindromic Repeats) to describe these structures. However, their biological significance remained a mystery.
Unraveling the Function of CRISPR
The turning point came in 2003, when Mojica and his team discovered that the spacer sequences between CRISPR repeats matched fragments of viral or plasmid DNA. This observation led him to hypothesize that CRISPR might serve as an adaptive immune system for bacteria, allowing them to "remember" and defend against viral infections. His hypothesis challenged conventional wisdom, as microbiologists had long believed that bacteria lacked an immune system akin to those found in higher organisms.
Mojica published his findings in 2005, proposing that CRISPR and its associated Cas (CRISPR-associated) proteins acted as a molecular defense mechanism. Bacteria, he argued, incorporated snippets of viral DNA into their own genomes, using these "mugshots" to recognize and destroy future viral invaders. The scientific community initially met Mojica’s theory with skepticism, but subsequent research—including work by Philippe Horvath and Rodolphe Barrangou—confirmed his predictions, validating the CRISPR-Cas system’s role in bacterial immunity.
From Basic Science to a Biotechnological Revolution
While Mojica’s work elucidated a fundamental biological process, it was the application of CRISPR-Cas9 as a gene-editing tool that catapulted the system into the global spotlight. Scientists like Doudna, Charpentier, and Feng Zhang refined the system, demonstrating its ability to precisely cut and modify DNA in any organism. This breakthrough earned them the 2020 Nobel Prize in Chemistry.
Despite not sharing the Nobel recognition, Mojica’s contributions remain foundational. His persistence in studying an obscure genetic element laid the groundwork for a technology now used to edit genomes, treat genetic disorders, engineer crops, and even combat infectious diseases. Mojica himself has expressed pride in his role, emphasizing the importance of curiosity-driven science in driving technological advancement.
Challenges and Ethical Considerations
The rapid adoption of CRISPR-Cas9 has raised important ethical questions about gene editing’s potential misuse. Mojica has spoken out on these issues, advocating for responsible innovation and regulatory oversight. He emphasizes that while CRISPR offers immense benefits—such as curing genetic diseases—its power necessitates careful consideration, particularly in human germline editing and ecological interventions.
Conclusion of Part One
Francisco Mojica’s story exemplifies the serendipitous nature of scientific discovery. His dedication to unraveling the mysteries of microbial genetics not only transformed our understanding of bacterial immunity but also ignited a biotechnological revolution. As CRISPR continues to reshape medicine, agriculture, and beyond, Mojica’s legacy serves as a testament to the enduring impact of foundational research.
In the next part of this article, we will delve deeper into Mojica’s scientific journey, the global recognition of his work, and his current research endeavors.
The Global Recognition of Francisco Mojica’s Work
While Francisco Mojica’s early work on CRISPR went largely unnoticed outside specialized microbiology circles, the scientific community gradually began to recognize its monumental importance. As researchers confirmed his hypothesis of CRISPR’s role in bacterial immunity, Mojica’s name became synonymous with one of the most significant biological discoveries of the modern era. Despite the later contributions of CRISPR-Cas9 pioneers like Jennifer Doudna and Emmanuelle Charpentier, Mojica’s foundational role has been increasingly celebrated. Awards, honors, and invitations to speak at major conferences cemented his status as a key figure in molecular biology.
Scientific Validation and Breakthrough Collaborations
In the years following Mojica’s 2005 paper, multiple research groups provided experimental evidence supporting CRISPR’s function as a bacterial immune defense. Scientists like Rodolphe Barrangou and Philippe Horvath at Danisco (a food-ingredient company) demonstrated in 2007 that CRISPR-Cas systems could immunize bacteria against bacteriophages—a direct validation of Mojica’s predictions. Their findings had immediate industrial applications, particularly in protecting bacterial cultures used in yogurt and cheese production from viral contamination.
Meanwhile, structural biologists and biochemists began deciphering how Cas proteins, particularly Cas9, functioned at a molecular level. By 2012, when Doudna, Charpentier, and Feng Zhang published their landmark papers on CRISPR-Cas9’s programmable gene-editing potential, the world quickly grasped the implications. Mojica’s early work was repeatedly cited as the cornerstone of this revolution. Though the Nobel Prize eluded him, Mojica received widespread recognition, including the 2017 Albany Medical Center Prize in Medicine and Biomedical Research (shared with Doudna, Charpentier, and others).
Overcoming Underfunding and Institutional Challenges
Mojica’s journey was not without obstacles. As a researcher at a Spanish public university, he faced chronic underfunding and a lack of resources compared to well-equipped labs in the U.S. or Northern Europe. For years, his team had to rely on ingenuity and perseverance rather than cutting-edge technology. In interviews, Mojica has described how he and his colleagues manually sequenced DNA fragments and painstakingly analyzed genetic data without the high-throughput tools available to better-funded institutions.
Despite these limitations, his insights were profound. The realization that CRISPR spacers matched viral DNA—a eureka moment that emerged from meticulous comparative genomics—was achieved with modest means. Mojica’s story highlights how curiosity-driven science, even in less prestigious or well-funded settings, can lead to transformative discoveries.
Mojica’s Perspective on the CRISPR Revolution
Unlike scientists who rapidly patented CRISPR applications for profit, Mojica has remained committed to fundamental research. He has often emphasized the importance of understanding CRISPR’s natural mechanisms before harnessing its power. In a 2016 interview, he remarked, "The beauty of CRISPR is that nature invented it. We are just borrowing it." This philosophy reflects his humility and dedication to biology for its own sake.
A Voice for Ethical Responsibility
As CRISPR technology advanced, Mojica became an advocate for ethical caution. He expressed concerns about premature human germline editing, particularly after the controversial 2018 case of He Jiankui, who claimed to have created the world’s first gene-edited babies. Mojica warned against prioritizing speed over safety, stating, "We have a responsibility to ensure that CRISPR is used for global benefit, not uncontrolled experimentation." He supports international regulations to prevent misuse while encouraging therapeutic applications for diseases like sickle-cell anemia and cystic fibrosis.
Patent Battles and the Cost of Discovery
The explosive commercial potential of CRISPR led to fierce patent disputes, primarily between the Broad Institute (Feng Zhang) and teams led by Doudna and Charpentier. Mojica, despite his foundational contributions, was not involved in these legal battles. Some scientists argued that he deserved a share of the intellectual property rights, given his role in discovering CRISPR’s function. However, Mojica has largely stayed out of financial disputes, focusing instead on advancing research.
Mojica’s Ongoing Research
Today, Mojica continues to investigate CRISPR systems at the University of Alicante. His lab explores new types of CRISPR-Cas variants, including lesser-known systems like Cas3 and Cas12, which may offer alternative gene-editing tools. He is particularly interested in archaeal CRISPR mechanisms, revisiting the extremophiles that first led him to uncover CRISPR’s existence.
New Frontiers: Beyond Cas9
While CRISPR-Cas9 remains the most widely used system, Mojica’s work suggests that other CRISPR-associated proteins could have untapped potential. For example, CRISPR-Cas12a (Cpf1) enables different editing patterns and could be more precise for certain applications. By studying diverse bacterial and archaeal species, Mojica hopes to uncover novel CRISPR variants with unique properties—ones that might circumvent some of the limitations or risks of Cas9.
CRISPR in Antibiotic Resistance and Environmental Adaptation
Another focus of Mojica’s current research is the role of CRISPR in bacterial evolution, particularly in antibiotic resistance. Some evidence suggests that CRISPR systems influence how bacteria acquire or lose resistance genes. Understanding these dynamics could lead to strategies for curbing the spread of superbugs. Additionally, Mojica investigates how environmental factors, such as extreme salinity or temperature, shape CRISPR diversity in microbial communities.
Public Engagement and Legacy
Despite his reserved demeanor, Mojica has embraced his role as a science communicator. He gives lectures worldwide, emphasizing the importance of basic research and perseverance. In Spain, he has become a symbol of scientific excellence, inspiring young researchers to pursue ambitious projects even without vast resources.
Recognition in Spain and Beyond
In 2017, Mojica received the Rey Jaime I Award for Basic Research, one of Spain’s highest scientific honors. The Spanish government has since increased funding for CRISPR research, partly due to his advocacy. Internationally, institutions like the Royal Society and the U.S. National Academy of Sciences have invited him as a keynote speaker, acknowledging his pivotal contributions.
Conclusion of Part Two
Francisco Mojica’s journey—from quietly studying salt-loving microbes to witnessing the global impact of his discovery—epitomizes the unpredictable nature of scientific progress. His work underscores the importance of fundamental research and intellectual humility. As CRISPR technology continues to evolve, Mojica remains both a guiding voice for responsible innovation and an active explorer of CRISPR’s unexplored frontiers.
In the final part of this article, we will examine Mojica’s influence on future biotechnology, his thoughts on the future of CRISPR, and how his legacy is shaping the next generation of scientists.
Francisco Mojica’s Legacy and the Future of CRISPR
As CRISPR gene-editing technologies move from laboratories to real-world applications, Francisco Mojica's contributions continue to shape the trajectory of modern biology. His journey represents more than a scientific breakthrough - it embodies how curiosity-driven research, often conducted far from traditional scientific power centers, can revolutionize our world. This final section explores Mojica's enduring impact on biotechnology, his vision for CRISPR's future, and how his legacy inspires new generations of scientists.
Democratizing Gene Editing Technology
One of CRISPR's most revolutionary aspects has been its accessibility. Unlike previous gene-editing techniques that required specialized expertise and expensive equipment, CRISPR protocols are relatively simple and low-cost. Mojica's foundational work helped enable this democratization of genetic engineering. Today, high school students conduct CRISPR experiments, startups emerge from garage labs, and researchers worldwide use the technology without patent restrictions for academic purposes.
Mojica has expressed particular enthusiasm about CRISPR's potential in developing nations. In interviews, he highlights agricultural applications that could help small farmers develop drought-resistant crops, or medical uses addressing diseases prevalent in tropical climates. "The most rewarding outcome would be seeing CRISPR improve lives in communities that traditional biotech has overlooked," he remarked in 2021.
Beyond Medicine: CRISPR's Expanding Horizons
Agricultural Transformations
While medical applications dominate CRISPR headlines, agriculture may represent the most immediately impactful use of the technology. Mojica's early observations of bacterial immunity now inform plant breeding techniques that could revolutionize food security. Researchers have already developed:
- Mushrooms that resist browning
- Wheat resistant to powdery mildew
- Drought-tolerant corn varieties
- Pigs resistant to PRRS virus
Unlike traditional GMOs that insert foreign DNA, CRISPR-edited crops often contain only minor, targeted changes to existing genes. Mojica has advocated for nuanced regulatory approaches that recognize this distinction, facilitating the adoption of gene-edited crops while maintaining safety standards.
Environmental and Conservation Applications
CRISPR's potential extends to ecological challenges. Scientists are exploring:
- Gene drives to control invasive species
- Corals modified to withstand warmer oceans
- Mosquitoes engineered to block malaria transmission
Mojica cautions that such applications require careful study of ecological impacts. He supports field trials with rigorous containment protocols, noting that while CRISPR offers powerful tools, "nature's complexity demands both enthusiasm and humility from scientists."
The Next Generation of CRISPR Technology
Moving Beyond Cutting: Base and Prime Editing
While CRISPR-Cas9 makes double-strand breaks in DNA, newer techniques like base editing and prime editing allow more precise changes without cutting both DNA strands. Mojica's lab monitors these developments closely, particularly how they relate to natural CRISPR systems. "What we see in laboratories today may already exist in nature," he notes. "By studying extremophiles and other microbes, we might discover CRISPR variants that outperform our current tools."
Epigenetic Editing and RNA Targeting
The CRISPR toolbox now includes modifications that alter gene expression without changing the underlying DNA sequence. These epigenetic applications could treat conditions where temporary modulation of genes is preferable to permanent edits. Mojica's research into diverse CRISPR-Cas systems suggests nature may hold many more such mechanisms waiting to be discovered.
Mentorship and Inspiring Future Scientists
Nurturing Talent at the University of Alicante
Despite international acclaim, Mojica remains committed to his home institution, mentoring graduate students and postdocs. His lab maintains a family-like atmosphere where curiosity drives research directions. Former students describe how Mojica encourages creative thinking while emphasizing rigorous methodology.
Promoting Spanish Science
As Spain's most prominent microbiologist, Mojica actively advocates for increased research funding and better scientific infrastructure. His success has helped change perceptions about Spanish science, proving that groundbreaking work can emerge outside traditional research hubs. The Spanish government now cites CRISPR research as a national scientific priority.
Reflections on Scientific Discovery
Lessons From the CRISPR Journey
Mojica's experience offers several key insights for the scientific community:
- The importance of pursuing obscure questions
- Value of careful, methodical observation
- Persistence in the face of skepticism
- Collaboration across disciplines
His two-decade study of what many considered "junk DNA" exemplifies how fundamental research without immediate applications can yield paradigm-shifting technologies.
Ethical Frontiers in the CRISPR Era
Ongoing Debates
As CRISPR applications expand, ethical questions multiply:
- Should we edit human embryos to eliminate genetic diseases?
- How should gene-edited crops be regulated?
- What controls should govern environmental releases of gene drives?
Mojica contributes to these discussions through organizations like the CRISPRcon forum, advocating for inclusive dialogues involving scientists, ethicists, and the public. He emphasizes that technological capabilities shouldn't alone dictate what applications society pursues.
Personal Life and Recognition
Despite his fame, Mojica maintains a quiet personal life in Alicante with his family. Colleagues describe him as modest, dedicated, and remarkably unchanged by his scientific celebrity. In 2022, the University of Alicante established the Mojica Center for CRISPR Research, ensuring his legacy will continue to shape the institution that nurtured his career.
Awards and Honors
Recent recognitions include:
- The 2023 International Microbiology Prize
- Honorary doctorates from five universities
- Spain's National Research Award
Conclusion: The Lasting Impact of a Scientific Pioneer
Francisco Mojica's story transcends biotechnology. It demonstrates how an individual scientist's curiosity can alter the course of medicine, agriculture, and our relationship with biology itself. From his initial observation of strange DNA repeats to the unfolding CRISPR revolution, Mojica's work continues affecting billions of lives.
As gene editing evolves from theoretical possibility to therapeutic reality, Mojica remains both an inspiration and a moral compass for the field. His insistence on asking fundamental biological questions, his commitment to ethical applications, and his dedication to mentoring all serve as models for how science should progress in the CRISPR era.
The full implications of Mojica's discovery may take decades to unfold, but one truth is already clear: In the history of biological science, few researchers have so profoundly changed our capabilities while maintaining his level of humility and scientific integrity. The CRISPR revolution is still in its early chapters, but Francisco Mojica has already secured his place as one of biology's great pioneers.
Unveiling CRISPR: The Revolutionary Tool Redefining Genetic Engineering
The dawn of the 21st century witnessed the emergence of a transformative technology that is reshaping the landscape of genetic engineering and molecular biology: CRISPR, an acronym for Clustered Regularly Interspaced Short Palindromic Repeats. At the heart of this innovation is an elegant, yet profoundly powerful, system that has equipped scientists with the ability to edit the genome with unprecedented precision, efficiency, and flexibility.
CRISPR, originally discovered as a part of the bacterial immune system defending against viral invaders, has since been adapted into a versatile tool that can target and modify almost any region of any genome. The technology's groundbreaking potential garnered global attention in 2012 when a pivotal paper by Jennifer Doudna and Emmanuelle Charpentier detailed how the CRISPR-Cas9 system could be harnessed as a genetic scalpel.
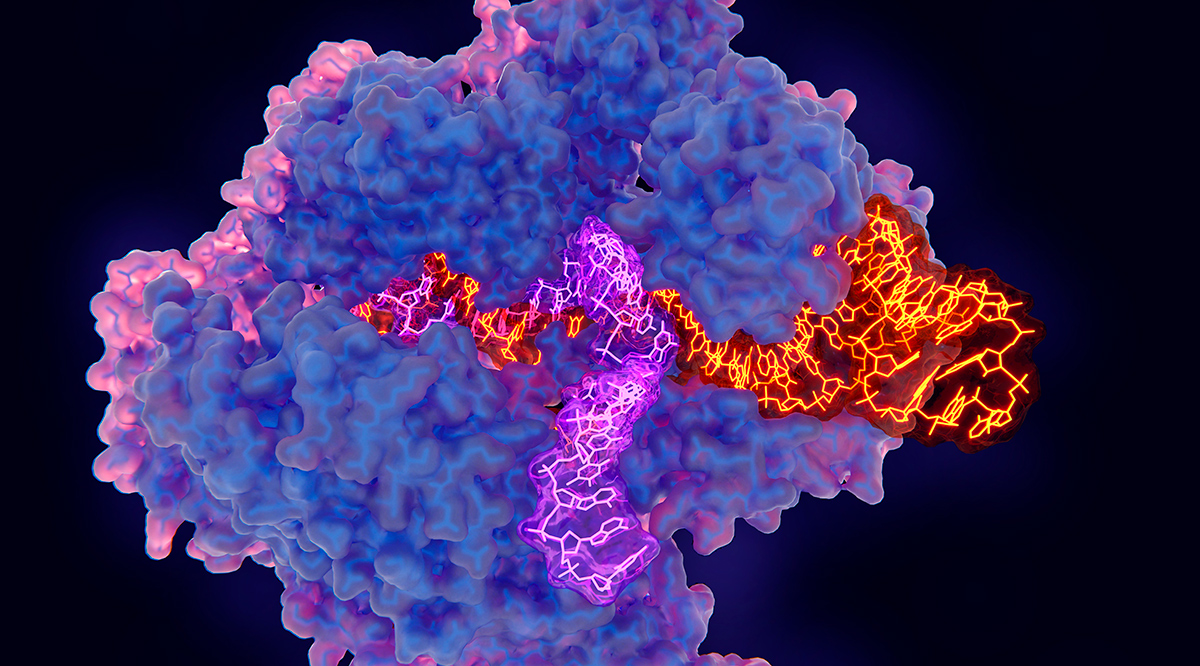
At its core, CRISPR functions as a two-component system. The first component is the Cas9 enzyme, a molecular scissor that can cut the double strands of DNA at specific sites. The second component is a guide RNA (gRNA), a piece of synthetic RNA designed to match the DNA sequence where the edit is intended. When both components are introduced into a cell, the guide RNA directs Cas9 to the precise DNA target, where it makes a cut. The cell's natural repair machinery then takes over, either knitting the cut back together, potentially inactivating a gene, or incorporating a new piece of DNA into the genome at the cut site, effectively rewriting the genetic code.
The implications of this innovation are immense. In the short period since its inception, CRISPR has been employed in a myriad of applications across various fields. In medicine, it offers hope for gene therapies that target and potentially cure hereditary diseases such as cystic fibrosis, sickle cell anemia, and hemophilia. In agriculture, CRISPR has been used to engineer crops with desirable traits like drought resistance and enhanced nutritional content, without incorporating foreign DNA, unlike traditional genetically modified organisms (GMOs).
However, CRISPR's extraordinary capabilities have also sparked intense ethical debates, especially pertaining to its use in human embryos. The prospect of 'designer babies' with traits selected by parents raises fundamental questions about the nature of human agency, consent, and the social implications of manipulating human genetics.
Despite the ongoing ethical and regulatory discussions, the potential of CRISPR technology has continued to expand drastically. Research and development have progressed from simple gene edits to more sophisticated genome manipulations, such as base editing, which allows the conversion of one DNA base into another without cutting the DNA strand. The most recent advancement, prime editing, promises to further refine the precision of genetic edits, broadening the scope of CRISPR's applications to possibly correct up to 89% of known genetic variations associated with human diseases.
Consequently, the future of CRISPR seems limitless. It stands as not only a pillar of contemporary genetic research but also a beacon of hope for tailored therapies and sustainable biotechnological innovations. Nonetheless, the excitement surrounding CRISPR must be calibrated with caution, as it not only molds the way we interact with the very fabric of life but also challenges our ethical thresholds and societal values.
As CRISPR continues to develop, questions about accessibility, safety, and governance remain at the forefront of the conversation. It promises to be a journey marked by incredible scientific advancements, as well as challenging socio-ethical deliberations. One thing is certain: CRISPR technology has ignited a revolution in science that we are only just beginning to comprehend. Its history is currently being written, and its chapters will undoubtedly influence the narrative of humanity for generations to come.### CRISPR: The Journey from Concept to Cure
As we delve deeper into the CRISPR narrative, it becomes essential to understand the ongoing journey from its conception to its potential role in providing cures for the previously incurable. Enthusiasm within the scientific community is at an all-time high as researchers race to translate CRISPR from a laboratory phenomenon into real-world solutions.
The power of CRISPR lies in its simplicity and flexibility, but the transition from bench to bedside is fraught with challenges. One of the primary obstacles is the mode of delivery. For CRISPR to correct genetic defects, the components must effectively reach the target cells in the human body. Researchers are actively exploring vectors, such as modified viruses, lipid nanoparticles, and even physical methods like microinjection, to safely and efficiently deliver CRISPR into the cells without eliciting adverse immune responses.
CRISPR's first applications in humans have focused on conditions amenable to ex vivo treatments, where cells can be edited outside the body and then re-introduced. A seminal example is the treatment of certain blood disorders, such as beta-thalassemia and sickle cell disease. Clinical trials are underway, and early results have been promising, signaling a monumental step forward in the use of genome editing for therapeutic purposes.
Another groundbreaking application is in the realm of cancer treatment. In oncology, experimentation with CRISPR is advancing the field of immunotherapy, particularly with the engineering of T-cells to better recognize and attack cancer cells. CRISPR is instrumental in enhancing the specificity and efficacy of these T-cells, potentially offering new hope to patients with difficult-to-treat tumors.
Moving beyond treatment, CRISPR offers innovative pathways for diagnostics as well. The CRISPR-Cas system's ability to locate specific DNA sequences has been leveraged to develop sensitive and rapid tests for detecting viruses, like SARS-CoV-2, the virus responsible for COVID-19. Such diagnostic tools are quick, accurate, and could very well revolutionize pathogen detection in the coming years.
Yet, perhaps the most significant, and most controversial, frontier of CRISPR technology is its application in germline editing, where changes to the DNA could be passed on to future generations. Such an approach has profound implications. It could eradicate hereditary conditions from a family line, but it also comes with the risks of unintended off-target effects and the broader implications for human evolution.
In 2018, a global outcry ensued when a Chinese scientist claimed to have produced the first humans—twin girls—whose genomes had been edited using CRISPR to confer resistance to HIV. This incident highlighted the need for stringent ethical guidelines and regulatory oversight, as the potential for misuse of this potent technology is a legitimate concern.
In response to such controversies, professional societies and regulatory agencies across the world are attempting to establish frameworks that permit safe and ethical research. Major initiatives include calls for international cooperation to develop consensus on governance and oversight, as well as fostering public engagement to understand societal perspectives on genome editing.
The CRISPR revolution has also sparked a patent battle, given the significant commercial and therapeutic implications of the technology. The foundational patents are currently held by the Broad Institute of MIT and Harvard in the United States, and by the University of California, Berkeley, where much of the early work on CRISPR-Cas9 was conducted. Given CRISPR's wide-reaching potential, it is paramount that these disputes do not hinder the availability of the technology to scientists and clinicians worldwide.
Looking ahead, it is undeniable that CRISPR-Cas systems will continue to evolve, presenting even more sophisticated tools for genetic manipulation. Among these are next-generation editors, capable of multiplex editing, enabling multiple edits across the genome simultaneously, and anti-CRISPR proteins, providing a safety switch to control genome editing activity.
As we continue to write the chapters of the CRISPR story, the narrative is not exclusively scientific or medical—it is inherently human. CRISPR has sown the seeds for a future where genetic diseases might be a thing of the past, where agriculture sustains an ever-growing global population, and where we might even reshape the very ecosystems we inhabit. But with great power comes great responsibility, and the journey of CRISPR is as much about exploring the potentials of human ingenuity as it is about navigating the ethical mazes that accompany our advancing capabilities.
In the vast and intricate tapestry that is the CRISPR saga, we find ourselves at a pivotal juncture, threading the fine line between possibility and prudence. As this revolutionary tool carves out its place in our collective history, one thing is certain: the CRISPR conversation is not just about genes; it's about our values, our future, and ultimately, our humanity.