Unveiling CRISPR: The Revolutionary Tool Redefining Genetic Engineering
The dawn of the 21st century witnessed the emergence of a transformative technology that is reshaping the landscape of genetic engineering and molecular biology: CRISPR, an acronym for Clustered Regularly Interspaced Short Palindromic Repeats. At the heart of this innovation is an elegant, yet profoundly powerful, system that has equipped scientists with the ability to edit the genome with unprecedented precision, efficiency, and flexibility.
CRISPR, originally discovered as a part of the bacterial immune system defending against viral invaders, has since been adapted into a versatile tool that can target and modify almost any region of any genome. The technology's groundbreaking potential garnered global attention in 2012 when a pivotal paper by Jennifer Doudna and Emmanuelle Charpentier detailed how the CRISPR-Cas9 system could be harnessed as a genetic scalpel.
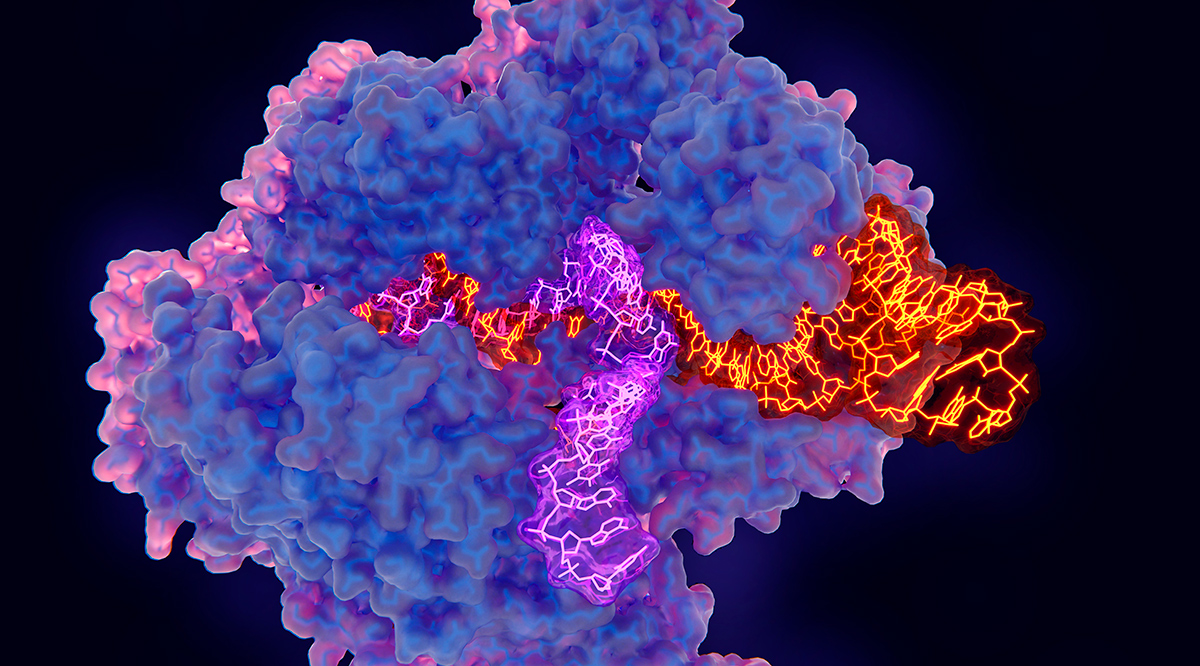
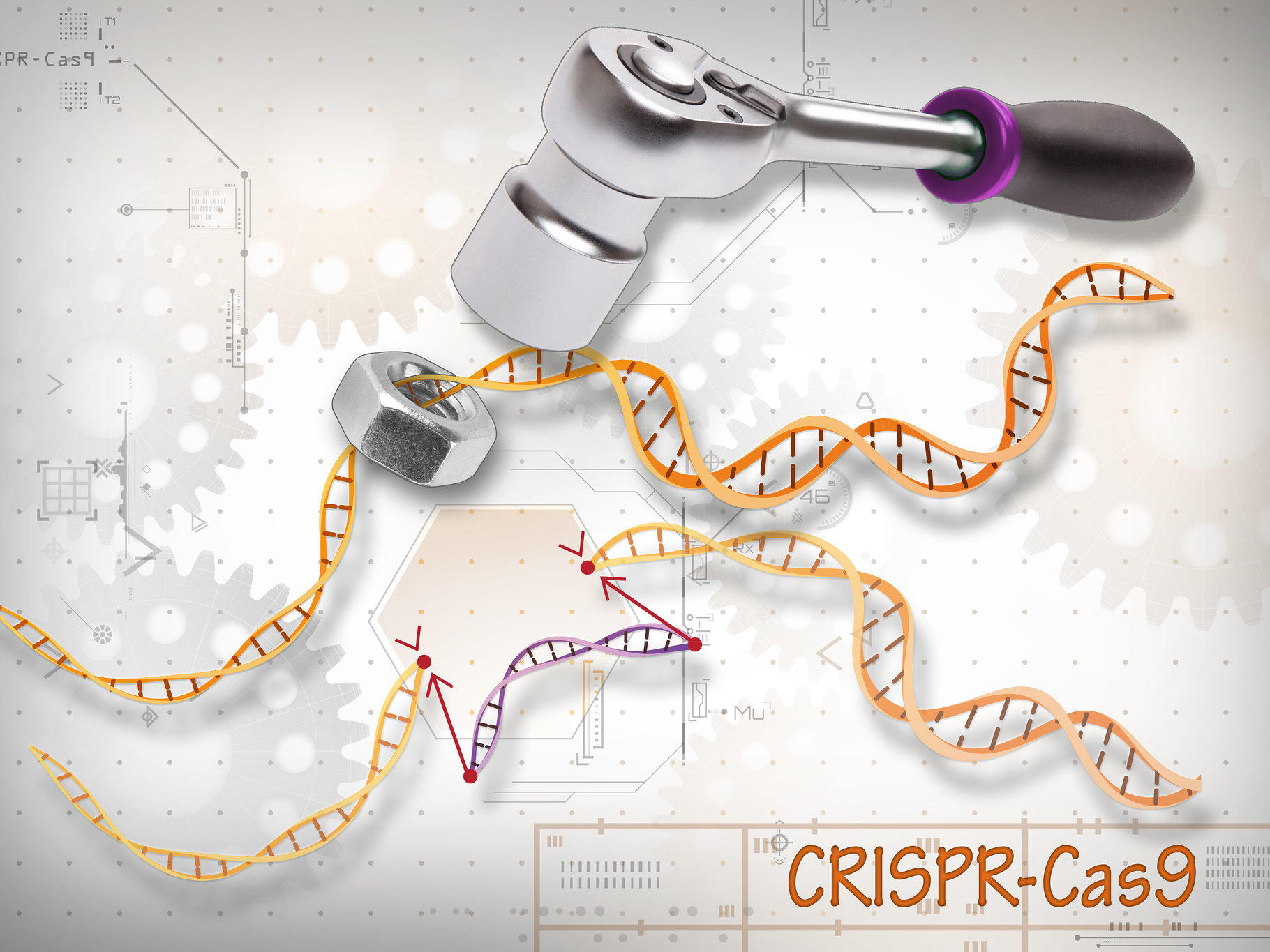
At its core, CRISPR functions as a two-component system. The first component is the Cas9 enzyme, a molecular scissor that can cut the double strands of DNA at specific sites. The second component is a guide RNA (gRNA), a piece of synthetic RNA designed to match the DNA sequence where the edit is intended. When both components are introduced into a cell, the guide RNA directs Cas9 to the precise DNA target, where it makes a cut. The cell's natural repair machinery then takes over, either knitting the cut back together, potentially inactivating a gene, or incorporating a new piece of DNA into the genome at the cut site, effectively rewriting the genetic code.
The implications of this innovation are immense. In the short period since its inception, CRISPR has been employed in a myriad of applications across various fields. In medicine, it offers hope for gene therapies that target and potentially cure hereditary diseases such as cystic fibrosis, sickle cell anemia, and hemophilia. In agriculture, CRISPR has been used to engineer crops with desirable traits like drought resistance and enhanced nutritional content, without incorporating foreign DNA, unlike traditional genetically modified organisms (GMOs).
However, CRISPR's extraordinary capabilities have also sparked intense ethical debates, especially pertaining to its use in human embryos. The prospect of 'designer babies' with traits selected by parents raises fundamental questions about the nature of human agency, consent, and the social implications of manipulating human genetics.
Despite the ongoing ethical and regulatory discussions, the potential of CRISPR technology has continued to expand drastically. Research and development have progressed from simple gene edits to more sophisticated genome manipulations, such as base editing, which allows the conversion of one DNA base into another without cutting the DNA strand. The most recent advancement, prime editing, promises to further refine the precision of genetic edits, broadening the scope of CRISPR's applications to possibly correct up to 89% of known genetic variations associated with human diseases.
Consequently, the future of CRISPR seems limitless. It stands as not only a pillar of contemporary genetic research but also a beacon of hope for tailored therapies and sustainable biotechnological innovations. Nonetheless, the excitement surrounding CRISPR must be calibrated with caution, as it not only molds the way we interact with the very fabric of life but also challenges our ethical thresholds and societal values.
As CRISPR continues to develop, questions about accessibility, safety, and governance remain at the forefront of the conversation. It promises to be a journey marked by incredible scientific advancements, as well as challenging socio-ethical deliberations. One thing is certain: CRISPR technology has ignited a revolution in science that we are only just beginning to comprehend. Its history is currently being written, and its chapters will undoubtedly influence the narrative of humanity for generations to come.### CRISPR: The Journey from Concept to Cure
As we delve deeper into the CRISPR narrative, it becomes essential to understand the ongoing journey from its conception to its potential role in providing cures for the previously incurable. Enthusiasm within the scientific community is at an all-time high as researchers race to translate CRISPR from a laboratory phenomenon into real-world solutions.
The power of CRISPR lies in its simplicity and flexibility, but the transition from bench to bedside is fraught with challenges. One of the primary obstacles is the mode of delivery. For CRISPR to correct genetic defects, the components must effectively reach the target cells in the human body. Researchers are actively exploring vectors, such as modified viruses, lipid nanoparticles, and even physical methods like microinjection, to safely and efficiently deliver CRISPR into the cells without eliciting adverse immune responses.
CRISPR's first applications in humans have focused on conditions amenable to ex vivo treatments, where cells can be edited outside the body and then re-introduced. A seminal example is the treatment of certain blood disorders, such as beta-thalassemia and sickle cell disease. Clinical trials are underway, and early results have been promising, signaling a monumental step forward in the use of genome editing for therapeutic purposes.
Another groundbreaking application is in the realm of cancer treatment. In oncology, experimentation with CRISPR is advancing the field of immunotherapy, particularly with the engineering of T-cells to better recognize and attack cancer cells. CRISPR is instrumental in enhancing the specificity and efficacy of these T-cells, potentially offering new hope to patients with difficult-to-treat tumors.
Moving beyond treatment, CRISPR offers innovative pathways for diagnostics as well. The CRISPR-Cas system's ability to locate specific DNA sequences has been leveraged to develop sensitive and rapid tests for detecting viruses, like SARS-CoV-2, the virus responsible for COVID-19. Such diagnostic tools are quick, accurate, and could very well revolutionize pathogen detection in the coming years.
Yet, perhaps the most significant, and most controversial, frontier of CRISPR technology is its application in germline editing, where changes to the DNA could be passed on to future generations. Such an approach has profound implications. It could eradicate hereditary conditions from a family line, but it also comes with the risks of unintended off-target effects and the broader implications for human evolution.
In 2018, a global outcry ensued when a Chinese scientist claimed to have produced the first humans—twin girls—whose genomes had been edited using CRISPR to confer resistance to HIV. This incident highlighted the need for stringent ethical guidelines and regulatory oversight, as the potential for misuse of this potent technology is a legitimate concern.
In response to such controversies, professional societies and regulatory agencies across the world are attempting to establish frameworks that permit safe and ethical research. Major initiatives include calls for international cooperation to develop consensus on governance and oversight, as well as fostering public engagement to understand societal perspectives on genome editing.
The CRISPR revolution has also sparked a patent battle, given the significant commercial and therapeutic implications of the technology. The foundational patents are currently held by the Broad Institute of MIT and Harvard in the United States, and by the University of California, Berkeley, where much of the early work on CRISPR-Cas9 was conducted. Given CRISPR's wide-reaching potential, it is paramount that these disputes do not hinder the availability of the technology to scientists and clinicians worldwide.
Looking ahead, it is undeniable that CRISPR-Cas systems will continue to evolve, presenting even more sophisticated tools for genetic manipulation. Among these are next-generation editors, capable of multiplex editing, enabling multiple edits across the genome simultaneously, and anti-CRISPR proteins, providing a safety switch to control genome editing activity.
As we continue to write the chapters of the CRISPR story, the narrative is not exclusively scientific or medical—it is inherently human. CRISPR has sown the seeds for a future where genetic diseases might be a thing of the past, where agriculture sustains an ever-growing global population, and where we might even reshape the very ecosystems we inhabit. But with great power comes great responsibility, and the journey of CRISPR is as much about exploring the potentials of human ingenuity as it is about navigating the ethical mazes that accompany our advancing capabilities.
In the vast and intricate tapestry that is the CRISPR saga, we find ourselves at a pivotal juncture, threading the fine line between possibility and prudence. As this revolutionary tool carves out its place in our collective history, one thing is certain: the CRISPR conversation is not just about genes; it's about our values, our future, and ultimately, our humanity.


















Comments