Gregor Johann Mendel: The Pioneer of Modern Genetics
Introduction to Gregor Mendel's Legacy
Gregor Johann Mendel, a 19th-century scientist, revolutionized our understanding of heredity through his meticulous experiments with pea plants. His work laid the foundation for modern genetics, introducing concepts like dominance, segregation, and independent assortment. Despite initial obscurity, Mendel's discoveries became cornerstones of biological science.
Early Life and Scientific Context
Born in 1822 in what is now the Czech Republic, Mendel entered the Augustinian monastery in Brno, where he combined his religious duties with scientific pursuits. The scientific context of his time was dominated by theories of blending inheritance, where traits were thought to merge between generations. Mendel's groundbreaking approach challenged these ideas.
Challenging the Status Quo
Unlike his contemporaries, Mendel believed in discrete hereditary factors—now known as genes. His experiments with Pisum sativum (pea plants) demonstrated that traits did not blend but were passed down in predictable patterns. This was a radical departure from the prevailing scientific thought.
Mendel's Groundbreaking Experiments
Between 1856 and 1863, Mendel conducted controlled crosses of pea plants, meticulously tracking seven distinct traits. His quantitative approach yielded consistent numerical ratios, such as the 3:1 ratio in monohybrid crosses, which revealed the principles of dominance and segregation.
The Scale of Mendel's Work
Mendel's experiments were unprecedented in their scale. He grew and recorded data from approximately 10,000 pea plants, ensuring statistical robustness. This large-scale approach allowed him to observe patterns that smaller studies might have missed.
Key Findings and Mendelian Ratios
Mendel's work identified several key ratios that became fundamental to genetics:
- 3:1 phenotypic ratio in the F2 generation of monohybrid crosses.
- 9:3:3:1 ratio in dihybrid crosses, illustrating independent assortment.
These ratios provided empirical evidence for his theories and remain central to genetic education today.
The Rediscovery of Mendel's Work
Despite publishing his findings in 1866, Mendel's work was largely ignored during his lifetime. It wasn't until 1900—34 years after his paper's publication—that his discoveries were rediscovered by Hugo de Vries, Carl Correns, and Erich von Tschermak. This rediscovery marked the birth of genetics as a scientific discipline.
Why Was Mendel Ignored?
Several factors contributed to the initial neglect of Mendel's work:
- His paper was published in an obscure journal.
- The scientific community was not yet ready to accept his radical ideas.
- Mendel's mathematical approach was ahead of its time.
However, once rediscovered, his work quickly gained recognition for its experimental rigor and predictive power.
Mendel's Methodological Innovations
Mendel's success can be attributed to his methodological innovations. He combined experimental design, controlled crosses, and quantitative analysis in a way that was unprecedented. His approach included:
- Tracking individual traits separately.
- Replicating experiments across large sample sizes.
- Using simple arithmetic to analyze results.
These practices made his findings robust and reproducible, setting a new standard for scientific inquiry.
The Importance of Quantitative Analysis
Mendel's use of quantitative analysis was revolutionary. By counting and categorizing traits, he transformed genetics from a qualitative observation into a quantitative science. This shift allowed for the discovery of predictable patterns and ratios, which became the bedrock of genetic research.
Conclusion of Part 1
Gregor Mendel's work with pea plants fundamentally changed our understanding of heredity. His discoveries of dominance, segregation, and independent assortment laid the groundwork for modern genetics. Despite initial obscurity, his methodological rigor and quantitative approach ensured that his contributions would eventually be recognized as foundational to the field.
In Part 2, we will delve deeper into the specifics of Mendel's experiments, the traits he studied, and the broader implications of his work for modern genetics.
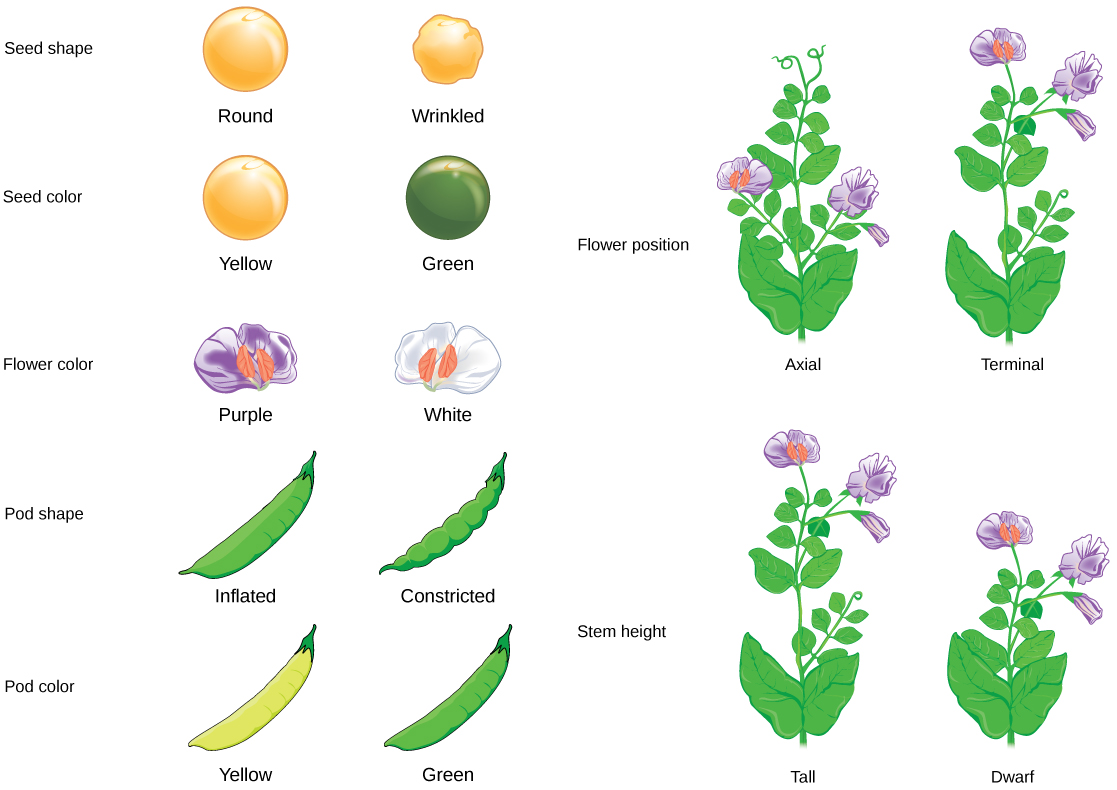
The Seven Traits That Defined Mendelian Genetics
Mendel's experiments focused on seven distinct traits in pea plants, each exhibiting clear dominance relationships. These traits were carefully chosen for their ease of observation and consistent inheritance patterns. By studying these characteristics, Mendel uncovered the fundamental principles of heredity.
Characteristics of the Seven Pea Plant Traits
The traits Mendel examined included:
- Flower color (purple vs. white)
- Flower position (axial vs. terminal)
- Stem length (tall vs. dwarf)
- Pod shape (inflated vs. constricted)
- Pod color (green vs. yellow)
- Seed shape (round vs. wrinkled)
- Seed color (yellow vs. green)
Each trait demonstrated a dominant-recessive relationship, where one form consistently appeared in the F1 generation while the other remained hidden, only to reappear in predictable ratios in the F2 generation.
Statistical Consistency Across Generations
Mendel's meticulous record-keeping revealed striking statistical patterns. For example, when crossing plants with purple flowers (dominant) and white flowers (recessive), the F1 generation uniformly displayed purple flowers. However, in the F2 generation, the ratio of purple to white flowers approximated 3:1—a pattern that held true across all seven traits.
Mendel's Laws: The Foundation of Heredity
From his experiments, Mendel derived three fundamental laws that govern inheritance:
The Law of Segregation
This law states that each individual possesses two alleles for a trait, which segregate during gamete formation. As a result, each parent contributes one allele to their offspring. Mendel observed this phenomenon when the recessive trait (e.g., white flowers) reappeared in the F2 generation after being absent in the F1 generation.
The Law of Dominance
The Law of Dominance explains why certain traits appear more frequently in offspring. Mendel found that one allele can mask the expression of another. For instance, the allele for purple flowers dominated over the allele for white flowers, causing the recessive trait to remain hidden in heterozygous individuals.
The Law of Independent Assortment
Mendel's dihybrid crosses—experiments tracking two traits simultaneously—led to the discovery of independent assortment. He observed that alleles for different traits are inherited independently of one another. This principle was evidenced by the 9:3:3:1 ratio in the F2 generation of dihybrid crosses, showing all possible combinations of the two traits.
Modern Validation and Exceptions to Mendel's Laws
While Mendel's laws remain foundational, modern genetics has revealed complexities that extend beyond his original observations. Advances in molecular genetics have validated his conceptual "factors" as genes located on chromosomes, but they have also identified exceptions to simple Mendelian inheritance.
Linkage and Genetic Recombination
One significant exception is linkage, where genes located close to one another on the same chromosome tend to be inherited together. This phenomenon was discovered by Thomas Hunt Morgan in the early 20th century. While Mendel's Law of Independent Assortment holds true for genes on different chromosomes, linked genes violate this principle due to their physical proximity.
Incomplete Dominance and Codominance
Not all traits exhibit the clear dominance relationships Mendel described. In cases of incomplete dominance, heterozygous individuals display a phenotype that is a blend of the two homozygous phenotypes. For example, crossing red and white snapdragons can yield pink offspring. Similarly, codominance occurs when both alleles are fully expressed in the phenotype, as seen in the AB blood type in humans.
Epistasis and Polygenic Inheritance
Epistasis occurs when one gene affects the expression of another gene. This interaction can produce unexpected phenotypic ratios that deviate from Mendel's predictions. Additionally, many traits are polygenic, meaning they are influenced by multiple genes. Examples include human height and skin color, which exhibit continuous variation rather than the discrete categories Mendel observed.
The Molecular Era: Identifying Mendel's Genes
Recent advancements in genomics have allowed scientists to identify the specific genes responsible for the traits Mendel studied. Research institutions, such as the John Innes Centre, have played a pivotal role in this endeavor, leveraging extensive pea germplasm collections to pinpoint the molecular basis of Mendel's phenotypic observations.
From Phenotype to Genotype
Modern techniques like DNA sequencing and gene mapping have enabled researchers to locate and characterize the genes underlying Mendel's seven traits. For instance:
- The gene for flower color has been identified as a transcription factor that regulates anthocyanin production.
- The stem length trait is controlled by a gene involved in gibberellin hormone synthesis.
- The seed shape gene affects starch branching enzyme activity, influencing seed texture.
These discoveries bridge the gap between Mendel's phenotypic observations and their genotypic foundations, providing a deeper understanding of inheritance at the molecular level.
Pea Germplasm Collections and Genetic Research
Institutions worldwide maintain vast pea germplasm collections, preserving thousands of Pisum sativum accessions. These resources are invaluable for genetic research, allowing scientists to study the diversity and evolution of Mendel's traits. For example, the John Innes Centre's collection includes historical varieties that Mendel himself might have used, offering a direct link to his groundbreaking experiments.
Mendel's Enduring Influence on Science and Education
Gregor Mendel's contributions extend far beyond his lifetime, shaping both scientific research and education. His principles of inheritance are taught in classrooms worldwide, serving as the bedrock of introductory genetics courses. Moreover, his methodological rigor continues to inspire scientists across disciplines.
Educational Impact: Teaching Mendelian Genetics
Mendel's experiments are a staple in biology education, illustrating key genetic concepts through accessible examples. Students learn about Punnett squares, which visually represent Mendel's principles of segregation and independent assortment. These tools help demystify inheritance patterns, making complex genetic ideas tangible and understandable.
Scientific Rigor and Experimental Design
Mendel's approach to scientific inquiry set a precedent for future researchers. His emphasis on controlled experiments, quantitative data, and reproducibility established a gold standard for experimental design. Today, these principles are integral to scientific methodology, ensuring that research is both reliable and valid.
Conclusion of Part 2
Gregor Mendel's work with pea plants unveiled the fundamental laws of inheritance, transforming our understanding of genetics. His discoveries of segregation, dominance, and independent assortment remain central to the field, even as modern genetics has uncovered additional complexities. The identification of the molecular basis for Mendel's traits further cements his legacy, bridging historical observations with contemporary science.
In Part 3, we will explore Mendel's personal life, the historical context of his work, and his lasting impact on both science and society. We will also examine how his discoveries continue to influence modern genetic research and biotechnology.
Gregor Mendel: The Man Behind the Science
While Gregor Mendel is celebrated for his scientific contributions, his personal life and the environment in which he worked are equally fascinating. Born Johann Mendel in 1822 in Heinzendorf, Austria (now Hynčice, Czech Republic), he adopted the name Gregor upon entering the Augustinian monastery in Brno. His journey from a rural background to becoming the father of modern genetics is a testament to his intellect and perseverance.
Early Life and Education
Mendel's early education was marked by financial struggles, but his academic potential was evident. He studied philosophy and physics at the University of Olomouc before entering the monastery. Later, he attended the University of Vienna, where he was exposed to scientific methods and mathematical principles that would later inform his genetic experiments.
Life in the Monastery
The Augustinian monastery in Brno provided Mendel with the resources and time to conduct his experiments. The monastery was a hub of intellectual activity, and Mendel's work was supported by his fellow monks. His role as a teacher and later as abbot allowed him to balance his scientific pursuits with his religious duties.
The Historical Context of Mendel's Discoveries
Mendel's work did not emerge in a vacuum; it was shaped by the scientific and cultural milieu of the 19th century. Understanding this context helps appreciate the significance of his contributions and why they were initially overlooked.
19th-Century Views on Heredity
Before Mendel, the prevailing theory of heredity was blending inheritance, which suggested that traits from parents blend together in offspring. This idea was challenged by Mendel's observation that traits could remain distinct and reappear in subsequent generations. His findings contradicted the dominant scientific narratives of his time, making them difficult for contemporaries to accept.
The Role of Mathematics in Biology
Mendel's use of statistical analysis was revolutionary in biology. At a time when biological studies were largely descriptive, his quantitative approach provided a new way to understand natural phenomena. This methodological innovation was ahead of its time and contributed to the initial neglect of his work, as many scientists were not yet prepared to embrace mathematical biology.
Mendel's Health and Later Years
Recent historical and genomic analyses have shed light on Mendel's later life and health. Studies suggest that he may have had a predisposition to heart disease, which could have influenced his work and longevity. Despite these challenges, Mendel remained active in his scientific and religious pursuits until his death in 1884.
Legacy and Posthumous Recognition
Mendel's work gained recognition only after his death, following its rediscovery in 1900. Today, he is celebrated as a pioneer of genetics, with numerous institutions and awards named in his honor. His experiments with pea plants are commemorated in museums and research centers, ensuring that his contributions are remembered and studied by future generations.
Mendel's Influence on Modern Genetics and Biotechnology
Mendel's principles of inheritance have had a profound impact on modern genetics and biotechnology. His discoveries laid the groundwork for advancements in molecular biology, genetic engineering, and personalized medicine. The understanding of gene inheritance has enabled breakthroughs in agriculture, healthcare, and beyond.
Applications in Agriculture
The principles of Mendelian genetics are fundamental to plant and animal breeding. By understanding dominance, segregation, and independent assortment, breeders can develop crops and livestock with desirable traits. This has led to improvements in yield, disease resistance, and nutritional value, addressing global food security challenges.
Advancements in Healthcare
In healthcare, Mendel's work has informed our understanding of genetic disorders and inheritance patterns. Conditions such as cystic fibrosis, sickle cell anemia, and Huntington's disease follow Mendelian inheritance patterns, allowing for better diagnosis, counseling, and treatment strategies. The field of genetic counseling relies heavily on Mendel's principles to assess the risk of inherited diseases.
Genetic Engineering and CRISPR
Modern biotechnology, including CRISPR gene editing, builds on the foundation laid by Mendel. By precisely manipulating genes, scientists can correct genetic defects, enhance crop traits, and even develop new therapies for previously untreatable conditions. Mendel's insights into gene inheritance have been instrumental in these advancements.
Commemorating Mendel: Museums and Research Institutes
Mendel's legacy is preserved and celebrated through various institutions dedicated to genetics and agricultural research. These centers not only honor his contributions but also continue his work, advancing our understanding of genetics and its applications.
The Gregor Mendel Museum
Located in Brno, the Gregor Mendel Museum is housed in the Augustinian monastery where Mendel conducted his experiments. The museum showcases his life, work, and the historical context of his discoveries. Visitors can explore the garden where Mendel grew his pea plants and learn about the impact of his research on modern science.
The John Innes Centre
The John Innes Centre in the UK is a leading research institution that has played a crucial role in identifying the molecular basis of Mendel's traits. Their extensive pea germplasm collections and cutting-edge research continue to uncover the genetic mechanisms underlying plant traits, building on Mendel's foundational work.
Conclusion: The Enduring Legacy of Gregor Mendel
Gregor Mendel's journey from a humble background to becoming the father of modern genetics is a story of curiosity, perseverance, and scientific rigor. His experiments with pea plants revealed the fundamental laws of inheritance, transforming our understanding of biology and laying the groundwork for countless advancements in science and technology.
Key Takeaways from Mendel's Work
Mendel's contributions can be summarized through several key takeaways:
- Discrete hereditary factors: Mendel's discovery of genes as distinct units of inheritance.
- Predictable inheritance patterns: The principles of segregation, dominance, and independent assortment.
- Quantitative approach: The importance of statistical analysis in biological research.
- Methodological rigor: The value of controlled experiments and reproducibility.
The Future of Genetics
As we continue to unravel the complexities of genetics, Mendel's principles remain a cornerstone of the field. From personalized medicine to genetic engineering, his work informs and inspires new generations of scientists. The ongoing research into the molecular basis of his traits ensures that Mendel's legacy will endure, shaping the future of biology and beyond.
In the words of Theodore Dobzhansky, "Nothing in biology makes sense except in the light of evolution." Similarly, nothing in genetics makes sense except in the light of Mendel's groundbreaking discoveries. His story is a reminder of the power of observation, experimentation, and the pursuit of knowledge—a legacy that continues to inspire and drive scientific progress.

Jacques Monod: Pionier der Molekularbiologie und Nobelpreisträger
Jacques Lucien Monod war ein französischer Biochemiker, dessen bahnbrechende Arbeit die Molekularbiologie grundlegend prägte. Für seine Entdeckungen zur genetischen Kontrolle von Enzymen erhielt er 1965 den Nobelpreis für Physiologie oder Medizin. Seine Modelle, wie das berühmte Operon-Modell, gelten noch heute als Meilensteine der modernen Genetik.
Frühes Leben und akademische Ausbildung
Jacques Monod wurde am 9. Februar 1910 in Paris geboren. Schon früh zeigte sich sein breites Interesse für Naturwissenschaften und Musik. Er begann sein Studium an der Universität Paris, wo er sich zunächst der Zoologie widmete. Seine wissenschaftliche Laufbahn wurde durch den Zweiten Weltkrieg unterbrochen, doch er promovierte dennoch im Jahr 1941.
Der Weg zum Pasteur-Institut
Ein entscheidender Wendepunkt war 1941 der Eintritt von Jacques Monod in das berühmte Pasteur-Institut in Paris. Hier fand er das ideale Umfeld für seine bahnbrechende Forschung. Ab 1945 übernahm er die Leitung der Abteilung für Mikroben-Physiologie und legte damit den Grundstein für seine späteren Nobelpreis-würdigen Entdeckungen.
Am Pasteur-Institut konzentrierte er seine Arbeit auf den Stoffwechsel von Bakterien, insbesondere von Escherichia coli. Diese Fokussierung erwies sich als äußerst fruchtbar und führte zur Entwicklung der Monod-Kinetik im Jahr 1949.
Die Monod-Kinetik: Ein Fundament der Biotechnologie
Im Jahr 1949 veröffentlichte Jacques Monod ein mathematisches Modell, das das Wachstum von Bakterienkulturen in Abhängigkeit von der Nährstoffkonzentration beschreibt. Dieses Modell, bekannt als Monod-Kinetik, wurde zu einem grundlegenden Werkzeug in der Mikrobiologie und Biotechnologie.
Die Formel erlaubt es, das mikrobielle Wachstum präzise vorherzusagen und zu steuern. Bis heute ist sie unverzichtbar in Bereichen wie der Fermentationstechnik, der Abwasserbehandlung und der industriellen Produktion von Antibiotika.
Die Monod-Kinetik beschreibt, wie die Wachstumsrate von Mikroorganismen von der Konzentration eines limitierenden Substrats abhängt – ein Prinzip, das in jedem biotechnologischen Labor Anwendung findet.
Entdeckung wichtiger Enzyme
Parallel zu seinen kinetischen Studien entdeckte und charakterisierte Monod mehrere Schlüsselenzyme. Diese Entdeckungen waren direkte Beweise für seine theoretischen Überlegungen zur Genregulation.
- Amylo-Maltase (1949): Ein Enzym, das am Maltose-Stoffwechsel beteiligt ist.
- Galactosid-Permease (1956): Ein Transporterprotein, das Lactose in die Bakterienzelle schleust.
- Galactosid-Transacetylase (1959): Ein Enzym mit Funktion im Lactose-Abbauweg.
Die Arbeit an diesen Enzymen führte Monod und seinen Kollegen François Jacob direkt zur Formulierung ihres revolutionären Operon-Modells.
Das Operon-Modell: Eine Revolution in der Genetik
Die gemeinsame Arbeit von Jacques Monod und François Jacob am Pasteur-Institut gipfelte in den frühen 1960er Jahren in der Entwicklung des Operon-Modells, auch Jacob-Monod-Modell genannt. Diese Theorie erklärte erstmals, wie Gene in Bakterien koordiniert reguliert und ein- oder ausgeschaltet werden.
Die Rolle der messenger-RNA
Ein zentraler Bestandteil des Modells war die Vorhersage der Existenz einer kurzlebigen Boten-RNA, der messenger-RNA (mRNA). Monod und Jacob postulierten, dass die genetische Information von der DNA auf diese mRNA kopiert wird, welche dann als Bauplan für die Proteinherstellung dient. Diese Vorhersage wurde kurz darauf experimentell bestätigt.
Die Entdeckung der mRNA war ein Schlüsselmoment für das Verständnis des zentralen Dogmas der Molekularbiologie und ist heute Grundlage für Technologien wie die mRNA-Impfstoffe.
Aufbau und Funktion des Lactose-Operons
Am Beispiel des Lactose-Operons in E. coli zeigten sie, dass strukturelle Gene, ein Operator und ein Promotor als eine funktionelle Einheit agieren. Ein Regulatorgen kodiert für ein Repressorprotein, das den Operator blockieren kann.
- Ohne Lactose bindet der Repressor am Operator und verhindert die Genexpression.
- Ist Lactose vorhanden, bindet sie an den Repressor, ändert dessen Form und löst ihn vom Operator.
- Die RNA-Polymerase kann nun die strukturellen Gene ablesen, und die Enzyme für den Lactoseabbau werden produziert.
Dieses elegante Modell der Genregulation erklärt, wie Zellen Energie sparen und sich flexibel an Umweltveränderungen anpassen können.
Die höchste wissenschaftliche Anerkennung: Der Nobelpreis 1965
Für diese bahnbrechenden Erkenntnisse wurde Jacques Monod zusammen mit François Jacob und André Lwoff im Jahr 1965 der Nobelpreis für Physiologie oder Medizin verliehen. Die offizielle Begründung des Nobelkomitees lautete: „für ihre Entdeckungen auf dem Gebiet der genetischen Kontrolle der Synthese von Enzymen und Viren“.
Die Verleihung dieses Preises markierte nicht nur den Höhepunkt von Monods Karriere, sondern unterstrich auch die zentrale Rolle des Pasteur-Instituts als globales Epizentrum der molekularbiologischen Forschung. Seine Arbeit hatte gezeigt, dass grundlegende Lebensprozesse auf molekularer Ebene verstanden und mathematisch beschrieben werden können.
Die Entdeckung des Operon-Modells war ein Paradigmenwechsel. Sie zeigte, dass Gene nicht einfach autonom funktionieren, sondern in komplexen Netzwerken reguliert werden.
Im nächsten Teil dieser Artikelserie vertiefen wir Monods Beitrag zur Allosterie-Theorie, seine philosophischen Schriften und sein bleibendes Vermächtnis für die moderne Wissenschaft.


Decoding Life: The Scientific Legacy of Sydney Brenner
Few scientists have shaped our understanding of life's fundamental processes like Sydney Brenner, a South African-born British biologist. As a central architect of modern molecular biology, Sydney Brenner made groundbreaking discoveries across genetics, developmental biology, and genomics. His work to decipher the genetic code and establish powerful model organisms created a blueprint for biological research that continues to guide scientists today.
The Architect of Molecular Biology's Golden Age
Sydney Brenner was a pivotal figure during what many call the golden age of molecular biology. His intellectual curiosity and collaborative spirit led to discoveries that answered some of the 20th century's most profound biological questions. Brenner's career was marked by a unique ability to identify crucial biological problems and pioneer the experimental tools needed to solve them.
Born in Germiston, South Africa, Brenner demonstrated exceptional scientific promise from a young age. He entered the University of Witwatersrand at just 14 years old and earned his medical degree. His quest for deeper biological understanding led him to Oxford University, where he completed his doctorate. This academic foundation set the stage for his historic contributions.
Brenner is widely recognized as one of the pioneers who presided over the golden age of molecular biology, establishing principles that enabled modern gene technology.
Groundbreaking Work in Cracking the Genetic Code
One of Sydney Brenner's earliest and most significant contributions was his work on deciphering the genetic code. After joining the prestigious Medical Research Council Laboratory of Molecular Biology in Cambridge, Brenner began collaborating with Francis Crick. Together, they tackled the mystery of how genetic information stored in DNA translates into functional proteins.
Proving the Triplet Nature of Codons
Brenner and Crick's collaboration produced a monumental breakthrough: proving that the genetic code is based on triplet codons. Through brilliant theoretical reasoning and experimentation, they demonstrated that a sequence of three nucleotides encodes a single amino acid. Brenner himself coined the essential term "codon" to describe these three-letter genetic words.
His work provided critical evidence against the theory of overlapping coding sequences. Brenner proved that the coding function of DNA was separate from its structural constraints, a fundamental concept in molecular genetics. This separation was essential for understanding how genetic information flows from genes to proteins.
Identifying the Stop Signal for Protein Synthesis
Beyond establishing the triplet code, Brenner made another crucial discovery. He identified a specific nonsense codon—the combination of uracil, adenine, and guanine—that signals the termination of protein translation. This discovery explained how cells know when to stop building a protein chain, completing our understanding of the genetic code's punctuation.
The impact of this work cannot be overstated. Cracking the genetic code provided the Rosetta Stone of molecular biology, allowing scientists to read and interpret the instructions within DNA. Brenner's contributions in this area alone would have secured his legacy, but he was only beginning his revolutionary scientific journey.
The Co-Discovery of Messenger RNA (mRNA)
While working on the genetic code, Sydney Brenner made another earth-shattering discovery with François Jacob and Matthew Meselson. In 1961, they proved the existence of messenger RNA (mRNA), solving a major mystery in molecular biology. Their experiments demonstrated that mRNA acts as a transient intermediate, carrying genetic instructions from DNA in the nucleus to the protein-making ribosomes in the cytoplasm.
This discovery filled a critical gap in the central dogma of molecular biology, which describes the flow of genetic information. Before Brenner's work, scientists struggled to understand exactly how DNA's information reached the cellular machinery that builds proteins. The identification of mRNA provided the missing link.
The significance of this breakthrough was immediately recognized by the scientific community. For his role in discovering messenger RNA, Brenner received the prestigious Albert Lasker Award for Basic Medical Research in 1971. This achievement highlights Brenner's extraordinary talent for identifying and solving foundational biological problems.
The discovery of messenger RNA was so significant that it earned Sydney Brenner the prestigious Albert Lasker Award for Basic Medical Research in 1971.
Establishing C. elegans: A Revolution in Biological Research
By the mid-1960s, with the genetic code essentially solved, Sydney Brenner deliberately shifted his research focus. He recognized that biology needed a new model organism to tackle the complexities of development and neurobiology. His visionary choice was the tiny, transparent roundworm Caenorhabditis elegans.
Why C. elegans Became the Perfect Model
Brenner selected C. elegans for several brilliant strategic reasons that demonstrated his deep understanding of experimental science:
- Genetic Simplicity: The worm has a small, manageable genome.
- Transparent Body: Researchers can observe cell division and development in living organisms under a microscope.
- Short Lifecycle: It completes its life cycle in just three days, enabling rapid genetic studies.
- Invariant Cell Lineage: Every worm develops identically, with exactly 959 somatic cells in the adult hermaphrodite.
Brenner's pioneering work proved that the worm's development—the timing, location, and fate of every cell division—was completely determined by genetics. He published his foundational paper, "The Genetics of Caenorhabditis elegans," in 1974, effectively creating an entirely new field of research.
The Transformational Impact of a Tiny Worm
The establishment of C. elegans as a model organism was arguably Brenner's most transformative contribution to biological science. This simple nematode became a powerful experimental system for investigating:
- Genetic regulation of organ development
- Programmed cell death (apoptosis)
- Nervous system structure and function
- Ageing and longevity
- Human disease mechanisms
Brenner succeeded in cloning most portions of the C. elegans DNA, creating essential tools for future researchers. His vision created a research paradigm that allowed scientists to study complex processes in a simple, genetically tractable animal. The choice of this model organism would ultimately lead to Nobel Prize-winning discoveries and continues to drive biomedical research today.
Genomics Pioneering and Vertebrate Model Development
Never content to rest on past achievements, Sydney Brenner continued to push scientific boundaries throughout his career. In the 1990s, he turned his attention to vertebrate genomics, recognizing the need for compact model genomes to advance genetic research. His innovative approach led to the introduction of an unusual but brilliant model organism: the pufferfish.
The Fugu Genome Project Breakthrough
Brenner introduced the pufferfish (Takifugu rubripes, commonly known as fugu) as a model vertebrate genome for comparative genomics. Despite being a vertebrate with complex biology similar to humans, the fugu has an exceptionally compact genome approximately 400 million base pairs in size. This is roughly eight times smaller than the human genome.
The compact nature of the fugu genome made it ideal for genetic studies. Brenner recognized that this streamlined DNA contained essentially the same genes as other vertebrates but with less non-coding "junk" DNA. This allowed researchers to identify functional elements and genes more efficiently than in larger, more complex genomes.
Brenner introduced the pufferfish as a model vertebrate genome, pioneering comparative genomics with its compact 400 million base pair genome.
Revolutionizing DNA Sequencing Technology
Sydney Brenner's contributions extended beyond biological discovery into technological innovation. He played a crucial role in advancing DNA sequencing methods that would eventually enable massive genomic projects. His work helped bridge the gap between early sequencing techniques and the high-throughput methods we rely on today.
Inventing Microbead Array-Based Sequencing
Brenner pioneered microbead array-based DNA sequencing technology, an approach that would influence future generations of sequencing platforms. This innovative method used microscopic beads to capture DNA fragments, allowing for parallel processing of multiple sequences simultaneously. This represented a significant step toward the high-throughput sequencing methods essential for modern genomics.
His work demonstrated the power of parallel processing in genetic analysis. By processing many DNA sequences at once, researchers could achieve unprecedented scale and efficiency. This approach foreshadowed the next-generation sequencing technologies that would later revolutionize biological research and medical diagnostics.
Commercial Applications and Lynx Therapeutics
Brenner's sequencing innovations found practical application through his work with Lynx Therapeutics. He collaborated with the company to develop massively parallel signature sequencing (MPSS), one of the first true next-generation sequencing methods. This technology could process millions of DNA fragments simultaneously, dramatically increasing sequencing capacity.
The MPSS system represented a quantum leap in sequencing capability. It utilized complex biochemical processes on microbeads to decode short DNA sequences in parallel. This work laid important groundwork for the DNA sequencing revolution that would follow in the 2000s, making large-scale genomic projects economically feasible.
Nobel Prize Recognition and Scientific Honors
The ultimate recognition of Sydney Brenner's scientific impact came in 2002 when he received the Nobel Prize in Physiology or Medicine. He shared this prestigious award with H. Robert Horvitz and John E. Sulston for their discoveries concerning "genetic regulation of organ development and programmed cell death."
The Nobel-Winning Research on Programmed Cell Death
The Nobel Committee specifically recognized Brenner's foundational work establishing C. elegans as a model organism for studying development. His colleagues Sulston and Horvitz had built upon this foundation to make crucial discoveries about programmed cell death (apoptosis). Their research revealed the genetic pathway that controls how and when cells deliberately die during development.
This Nobel Prize highlighted the far-reaching implications of Brenner's decision to work with C. elegans. The discoveries about cell death regulation have profound implications for understanding cancer, autoimmune diseases, and neurodegenerative disorders. When apoptosis fails to function properly, cells may multiply uncontrollably or fail to die when they should.
In 2002, Sydney Brenner shared the Nobel Prize in Physiology or Medicine for discoveries concerning genetic regulation of organ development and programmed cell death.
Additional Prestigious Awards and Recognition
Beyond the Nobel Prize, Brenner received numerous other honors throughout his distinguished career. These awards reflect the breadth and depth of his scientific contributions across multiple domains of biology:
- Albert Lasker Award for Basic Medical Research (1971) for the discovery of messenger RNA
- Royal Medal from the Royal Society (1974) for his contributions to molecular biology
- Gairdner Foundation International Award (1991) recognizing his outstanding biomedical research
- King Faisal International Prize in Science (1992) for his genetic research
- Copley Medal (2017) from the Royal Society, its oldest and most prestigious award
Brenner was elected to numerous prestigious academies, including the Royal Society, the National Academy of Sciences, and Germany's national academy of sciences, the Leopoldina. These memberships reflected the international recognition of his scientific leadership and the global impact of his research.
Leadership in Scientific Institutions and Mentorship
Throughout his career, Sydney Brenner demonstrated exceptional leadership in shaping scientific institutions and mentoring future generations of researchers. His vision extended beyond his own laboratory work to creating environments where innovative science could flourish.
The Molecular Sciences Institute in Berkeley
In 1995, Brenner founded the Molecular Sciences Institute in Berkeley, California with support from the Philip Morris Company. He sought to create an unconventional research environment where young scientists could pursue ambitious projects with intellectual freedom. The institute reflected Brenner's belief in supporting creative, boundary-pushing science without excessive bureaucratic constraints.
Brenner led the Institute until his retirement in 2000, establishing it as a center for innovative biological research. His leadership philosophy emphasized scientific independence and intellectual rigor. He believed that the best science emerged when talented researchers had the freedom to follow their scientific curiosity wherever it led.
Later Career at the Salk Institute
After retiring from the Molecular Sciences Institute, Brenner was appointed a Distinguished Professor at the Salk Institute in La Jolla, California. This appointment brought him full circle, reuniting him with his longtime collaborator Francis Crick, who had also joined the Salk Institute. Their renewed collaboration continued until Crick's death in 2004.
At Salk, Brenner continued to contribute his immense knowledge and experience to the scientific community. He maintained an active interest in emerging fields and technologies, always looking toward the future of biological research. His presence at Salk provided invaluable mentorship to younger scientists and continued his legacy of scientific excellence.
Scientific Philosophy and Approach to Research
Sydney Brenner's extraordinary scientific output was guided by a distinctive philosophy and approach to research. His methods and mindset offer valuable lessons for scientists across all disciplines.
The Importance of Choosing the Right Problem
Brenner was legendary for his ability to identify fundamental biological problems that were both important and solvable. He often emphasized that asking the right question was more important than having the right answer to the wrong question. This strategic approach to problem selection allowed him to make contributions that transformed entire fields.
His decision to switch from genetic code research to developmental biology demonstrated this philosophy perfectly. Having essentially solved the coding problem, he deliberately moved to what he saw as the next great challenge in biology: understanding multicellular development. This strategic shift led to his most influential work with C. elegans.
Innovation in Experimental Design
Brenner's innovative spirit extended to his experimental approaches. He consistently developed or adapted new methods to answer his scientific questions. From establishing C. elegans as a model organism to pioneering new sequencing technologies, Brenner understood that scientific progress often required methodological innovation.
His work demonstrates the importance of creating the right tools for the job. Rather than being limited by existing techniques, Brenner frequently invented new approaches when necessary. This willingness to innovate methodologically was a key factor in his ability to make breakthrough discoveries across multiple areas of biology.
The Enduring Scientific Legacy of Sydney Brenner
Sydney Brenner's impact on biological science extends far beyond his specific discoveries. His work established foundational principles that continue to guide research across multiple disciplines. Brenner's legacy includes not only what he discovered, but how he approached scientific problems and the tools he created for future generations.
The establishment of C. elegans as a model organism alone has generated an entire research ecosystem. Thousands of laboratories worldwide continue to use this tiny worm to study fundamental biological processes. Brenner's vision created a research paradigm that has produced multiple Nobel Prizes and countless scientific breakthroughs.
Impact on Modern Biomedical Research
Brenner's contributions directly enabled advances in understanding human disease mechanisms. The genetic pathways discovered in C. elegans have proven remarkably conserved in humans. Research on programmed cell death has led to new cancer treatments that target apoptosis pathways.
His work on the genetic code and mRNA laid the foundation for modern biotechnology and pharmaceutical development. Today's mRNA vaccines and gene therapies stand on the foundation Brenner helped build. The sequencing technologies he pioneered enable personalized medicine and genetic diagnostics.
Brenner's Influence on Scientific Culture and Education
Beyond his research achievements, Sydney Brenner shaped scientific culture through his mentorship and scientific communication. He trained numerous scientists who themselves became leaders in their fields. His approach to science emphasized creativity, intellectual courage, and collaboration.
Mentorship and Training Future Leaders
Brenner's laboratory served as a training ground for many prominent biologists. His mentorship style combined high expectations with generous intellectual freedom. He encouraged young scientists to pursue ambitious questions and develop their own research directions.
Many of his trainees have described how Brenner's guidance shaped their scientific careers. He emphasized the importance of scientific intuition and creative problem-solving. His legacy includes not only his discoveries but the generations of scientists he inspired and trained.
Scientific Communication and Writing
Brenner was known for his clear, often witty scientific writing and presentations. His ability to explain complex concepts in accessible terms made him an effective communicator. He wrote extensively about the philosophy of science and the future of biological research.
His famous "Life Sentences" columns in Current Biology showcased his talent for synthesizing complex ideas. These writings demonstrated his broad knowledge and his ability to connect disparate fields of science. Brenner's communication skills helped shape how molecular biology is taught and understood.
Brenner is widely recognized as one of the pioneers who presided over the golden age of molecular biology, establishing principles that enabled modern gene technology.
Brenner's Later Years and Final Contributions
Even in his later career, Sydney Brenner remained actively engaged with scientific developments. He continued to attend conferences, mentor younger scientists, and contribute to scientific discussions. His perspective as one of the founders of molecular biology gave him unique insights into the field's evolution.
Continued Scientific Engagement
Brenner maintained his characteristic curiosity throughout his life. He followed developments in genomics, neuroscience, and computational biology with keen interest. His ability to see connections between different scientific domains remained sharp until his final years.
He continued to offer valuable perspectives on the direction of biological research. Brenner often commented on emerging technologies and their potential impact. His experience allowed him to distinguish between fleeting trends and truly transformative developments.
Recognition and Honors in Later Life
In his final decades, Brenner received numerous additional honors recognizing his lifetime of achievement. These included the 2002 Nobel Prize and the Royal Society's Copley Medal in 2017. These late-career recognitions underscored the enduring significance of his contributions.
The scientific community continued to celebrate his work through special symposia and dedicated issues of scientific journals. These events brought together scientists whose work built upon Brenner's foundational discoveries. They demonstrated how his influence continued to shape biological research.
The Philosophical Underpinnings of Brenner's Approach
Sydney Brenner's scientific philosophy represented a unique blend of rigorous methodology and creative thinking. His approach to research offers enduring lessons for scientists across all disciplines.
The Importance of Simple Model Systems
Brenner's most profound insight may have been his recognition that complex biological problems often require simple experimental systems. His choice of C. elegans demonstrated that understanding basic principles in simple organisms could illuminate human biology. This approach has become central to modern biomedical research.
He understood that biological complexity could be best unraveled by studying systems where variables could be controlled. This philosophy has guided the development of model organisms from yeast to zebrafish. Brenner proved that simplicity could be the key to understanding complexity.
Interdisciplinary Thinking
Brenner's work consistently crossed traditional disciplinary boundaries. He moved seamlessly between genetics, biochemistry, developmental biology, and computational science. This interdisciplinary approach allowed him to see connections that specialists might miss.
His career demonstrates the power of synthesis across fields. Brenner's ability to incorporate insights from different domains enabled his most creative work. This approach has become increasingly important as biology becomes more integrated with physics, engineering, and computer science.
Quantifying Brenner's Scientific Impact
The scale of Sydney Brenner's influence can be measured through various metrics that demonstrate his extraordinary impact on biological science.
Citation Impact and Scientific Publications
Brenner's publications have been cited tens of thousands of times, with several papers achieving classic status. His 1974 paper "The Genetics of Caenorhabditis elegans" alone has been cited over 5,000 times. This paper essentially created an entire field of research that continues to grow.
His work on messenger RNA and the genetic code generated foundational papers that are still referenced today. The enduring relevance of his publications demonstrates how his work established principles that remain central to molecular biology.
Nobel Prize Legacy and Scientific Lineage
The Nobel Prize Brenner shared in 2002 was just one indicator of his impact. More significantly, his work directly enabled at least two additional Nobel Prizes awarded to scientists who built upon his foundations. The C. elegans system he created has been described as a "Nobel Prize factory."
His scientific lineage extends through multiple generations of researchers. Many prominent biologists today can trace their intellectual ancestry back to Brenner's laboratory. This scientific genealogy represents one of the most meaningful measures of his lasting influence.
Conclusion: The Enduring Legacy of a Scientific Visionary
Sydney Brenner's career represents one of the most productive and influential in the history of biological science. His contributions span the foundational discoveries of molecular biology's golden age to the genomic revolution of the 21st century. Brenner exemplified the combination of deep theoretical insight and practical experimental innovation.
His work established fundamental principles that continue to guide biological research. The genetic code, messenger RNA, model organism genetics, and DNA sequencing technologies all bear his distinctive imprint. Brenner's ability to identify crucial problems and develop innovative solutions set a standard for scientific excellence.
The most remarkable aspect of Brenner's legacy may be its continuing expansion. Each year, new discoveries build upon the foundations he established. The C. elegans system he created continues to yield insights into human biology and disease. The sequencing technologies he helped pioneer enable new approaches to medicine and research.
Sydney Brenner demonstrated that scientific progress depends on both brilliant discovery and the creation of tools for future discovery. His career reminds us that the most important scientific contributions are those that enable further exploration. Through his work and the generations of scientists he inspired, Brenner's influence will continue to shape biology for decades to come.
His life's work stands as a testament to the power of curiosity, creativity, and courage in scientific pursuit. Sydney Brenner not only decoded life's fundamental processes but also showed us how to ask the questions that matter most. This dual legacy ensures his permanent place among the greatest scientists of any generation.

Unveiling the Odyssey of François Jacob and Morphobioscience
The scientific journey of François Jacob represents a profound odyssey of discovery that reshaped modern biology. This article explores the revelation and narrativization of his pioneering research and its deep connections to the evolving history of morphobioscience. We will trace the path from his Nobel-winning insights to the broader implications for understanding life's complex architecture.
The Life and Legacy of François Jacob: A Scientific Pioneer
François Jacob was a French biologist whose collaborative work fundamentally altered our understanding of genetic regulation. Born in 1920, his life was marked by resilience, having served as a medical officer in the Free French Forces during World War II before turning to research. Alongside Jacques Monod and André Lwoff, he unveiled the operon model of gene control in bacteria.
This groundbreaking discovery earned them the 1965 Nobel Prize in Physiology or Medicine. Their work explained how genes could be switched on and off, a concept central to all biological development. Jacob's contributions extended beyond the operon, deeply influencing developmental biology and embryonic morphogenesis.
"The dream of every cell is to become two cells." - François Jacob
From War to the Laboratory: Jacob's Unlikely Path
Jacob's scientific career began after severe injury during the war redirected his path from surgery to research. His entry into the Pasteur Institute in 1950 placed him at the epicenter of a molecular biology revolution. This transition from medicine to fundamental research was crucial, providing a unique perspective on biological systems.
His wartime experiences cultivated a strategic mindset that he later applied to scientific problems. This background fostered a relentless drive to uncover the logical systems governing life, framing biology as an exercise in decoding complex information networks.
Deciphering the Operon: A Foundational Biological Narrative
The operon model stands as one of the most elegant narratives in modern science. Jacob and Monod proposed that clusters of genes could be regulated by a single operator switch. This model provided the first clear molecular logic for cellular differentiation and adaptation.
It answered a pivotal question: how do simple organisms manage complex behaviors? The discovery demonstrated that genes are not simply independent blueprints but are organized into functional, regulated circuits. This concept became a cornerstone for the emerging field of systems biology.
- The Lactose Operon (lac operon): The specific system studied, explaining how E. coli bacteria switch to consuming lactose when glucose is absent.
- Regulator Genes: These genes produce repressor proteins that can block transcription.
- The Operator Region: A DNA segment where the repressor binds, acting as the genetic "switch."
- Structural Genes: The cluster of genes expressed together when the operator switch is "on."
The Impact on Genetic and Embryological Thought
The operon model transcended bacterial genetics, offering a powerful metaphor for development in higher organisms. It suggested that the unfolding of form in an embryo could be directed by timed cascades of gene activation and repression. Jacob later became deeply interested in how these genetic circuits could orchestrate the complex morphogenesis of multicellular life.
This bridge between gene regulation and physical form is a key intersection with morphobioscience. Jacob's work implied that morphology is not pre-formed but computed in real-time by genomic networks. His ideas prompted biologists to reconsider embryos as self-organizing systems driven by regulated gene expression.
Exploring Morphobioscience: The Study of Biological Form
Morphobioscience is an integrative field concerned with the origin, development, and maintenance of biological form. It synthesizes concepts from embryology, evolution, genetics, and biophysics. The field seeks to understand how genetic information translates into three-dimensional structure and function.
This discipline moves beyond mere description of forms to explain the generative processes that create them. It asks not just "what does it look like?" but "how did it come to be shaped this way?" The history of this field is intertwined with the molecular revelations provided by researchers like François Jacob.
The Historical Trajectory of Form Studies
The history of studying biological form is long and rich, from Aristotle's observations to the comparative anatomy of the 19th century. The 20th century introduced two transformative paradigms: Darwinian evolution and molecular genetics. Jacob's work helped fuse these paradigms by providing a mechanism.
He showed how genetic changes in regulatory systems could produce altered forms upon which natural selection could act. This created a more complete narrative of evolutionary change, linking DNA sequence variation to phenotypic innovation. It addressed a critical gap in the Modern Synthesis of evolutionary biology.
Modern morphobioscience now employs advanced tools like live-cell imaging and computational modeling. These technologies allow scientists to visualize and simulate the dynamic processes of form generation that Jacob's theories helped to conceptualize.
The Interconnection: Jacob's Ideas and Morphobioscientific Philosophy
François Jacob's later writings, particularly his book "The Logic of Life," reveal his deep philosophical engagement with biological form. He argued that evolution works like a "tinkerer" (bricoleur), not an engineer. This metaphor suggests that new forms arise from modifying and recombining existing systems, not designing from scratch.
This concept is central to morphobioscience's understanding of evolutionary innovation. Most new anatomical structures are not wholly novel but are repurposed versions of old ones. The genetic regulatory networks Jacob discovered are the tools of this evolutionary tinkering.
His perspective encourages scientists to look for deep homologies—shared genetic circuitry underlying seemingly different forms in diverse species. This approach has been spectacularly confirmed in discoveries like the role of Hox genes in patterning animal bodies from insects to humans.
Evolution behaves like a tinkerer who, during eons upon eons, slowly reshapes his work. - François Jacob
The Narrative of Development as a Genetic Program
Jacob introduced the powerful, though sometimes debated, concept of the "genetic program." He described embryonic development as the execution of a coded plan contained within the DNA sequence. This narrative provided a framework for morphobioscience to interpret development as an informational process.
While modern science recognizes the crucial roles of physical forces and self-organization, the program metaphor was instrumental. It directed research toward deciphering the regulatory codes that coordinate cellular behavior in space and time. This quest continues to be a major driver in developmental biology and morphobioscience today.
Modern Morphobioscience: Beyond the Genetic Blueprint
The field of morphobioscience has advanced significantly beyond the initial metaphor of a simple genetic blueprint. While François Jacob's work on genetic regulation provided a foundational framework, contemporary research recognizes the immense complexity of emergent properties in biological form. Today, scientists integrate genetics with principles from physics, chemistry, and computational modeling to understand how forms self-assemble.
This evolution reflects a shift from a purely deterministic view to one that appreciates stochastic processes and self-organization. The development of an organism is now seen as a dialogue between its genetic instructions and the physical environment in which it grows. This more nuanced understanding is a direct descendant of the systems-thinking pioneered by Jacob and his contemporaries.
The Role of Physical Forces in Shaping Form
A key revelation in modern morphobioscience is the active role of biomechanical forces in development. Genes do not act in a vacuum; they produce proteins that alter cell adhesion, stiffness, and motility. These changes generate physical pressures and tensions that directly sculpt tissues, guiding the folding of an embryo's brain or the branching of its lungs.
This process, often called mechanotransduction, creates a feedback loop where form influences gene expression, which in turn alters form. It demonstrates that morphology is not a one-way street from gene to structure but a dynamic, reciprocal process. Understanding these forces is crucial for fields like regenerative medicine, where scientists aim to grow functional tissues in the lab.
- Cell Adhesion: Variations in how tightly cells stick together can cause sheets of tissue to buckle and fold, creating intricate structures.
- Cortical Tension: Differences in surface tension between cells can drive them to sort into specific layers, a fundamental step in organizing the early embryo.
- Matrix Mechanics: The stiffness or softness of the surrounding extracellular matrix can dictate whether a stem cell becomes bone, muscle, or nerve.
The Legacy of Jacob's "Tinkerer" in Evolutionary Developmental Biology (Evo-Devo)
The concept of evolution as a "tinkerer" has found its most powerful expression in the field of Evolutionary Developmental Biology, or Evo-Devo. This discipline explicitly seeks to understand how changes in developmental processes generate the evolutionary diversity of form. Jacob's insight that evolution works by modifying existing structures rather than inventing new ones from scratch is a central tenet of Evo-Devo.
By comparing the genetic toolkits used in the development of different animals, scientists have discovered profound similarities. The same families of genes that orchestrate the body plan of a fruit fly are used to pattern the body of a human, demonstrating a deep evolutionary homology. This provides concrete evidence for Jacob's narrative of evolutionary tinkering at the molecular level.
"The dream of the cell is to become two cells. The dream of the modern Evo-Devo researcher is to understand how a shared genetic toolkit builds a worm, a fly, and a human."
Hox Genes: The Master Regulators of Body Architecture
Perhaps the most stunning confirmation of Jacob's ideas came with the discovery of Hox genes. These are a set of regulatory genes that act as master switches, determining the identity of different segments along the head-to-tail axis of an animal. They are a quintessential example of a genetic module that has been copied, modified, and reused throughout evolution.
In a vivid illustration of tinkering, the same Hox genes that specify the thorax of an insect are used to pattern the mammalian spine. Variations in the expression patterns and targets of these genes contribute to the vast differences in body morphology between species. The study of Hox genes directly connects the molecular logic of the operon to the macroscopic evolution of animal form.
- Conservation: Hox genes are found in almost all animals and are arranged in clusters on the chromosome, a layout that is crucial to their function.
- Colinearity: The order of the genes on the chromosome corresponds to the order of the body regions they influence, a remarkable feature that underscores their role as a positional code.
- Modularity: Changes in Hox gene regulation can lead to major morphological innovations, such as the transformation of legs into antennae or the evolution of different limb types.
Morphobioscience in the 21st Century: Data, Imaging, and Synthesis
The 21st century has ushered in a new era for morphobioscience, driven by high-throughput technologies. The ability to sequence entire genomes, map all gene expression in a developing tissue, and image biological processes in real-time has generated vast datasets. The challenge is no longer acquiring data but synthesizing it into a coherent understanding of form.
This has led to the rise of computational morphodynamics, where researchers create mathematical models to simulate the emergence of form. These models integrate genetic, molecular, and physical data to test hypotheses about how complex structures arise. They represent the ultimate synthesis of the narratives started by Jacob—blending the logic of genetic programs with the dynamics of physical systems.
Live Imaging and the Dynamics of Development
Advanced microscopy techniques now allow scientists to watch development unfold live, capturing the dynamic cell movements that shape an embryo. This has transformed morphobioscience from a static, descriptive science to a dynamic, analytical one. Researchers can now observe the precise consequences of manipulating a gene or a physical force in real-time.
For example, watching neural crest cells migrate or observing the folds of the cerebral cortex form provides direct insight into the morphogenetic processes that Jacob could only infer. This technology directly tests his hypotheses about the temporal sequence of events in building biological form and has revealed a stunning level of plasticity and adaptability in developing systems.
The integration of live imaging with genetic manipulation and biophysical measurements is creating a more complete picture than ever before. It confirms that the narrative of morphogenesis is written not just by genes, but by the constant interplay between molecular signals and physical forces within a three-dimensional space.
Synthetic Biology and the Future of Designed Morphology
The principles uncovered by François Jacob and advanced by morphobioscience are now being actively applied in the field of synthetic biology. This discipline aims not just to understand life's design but to engineer it. Scientists are using the logic of genetic circuits—concepts directly descended from the operon model—to program cells with new functions and even new forms.
This represents a profound shift from analysis to synthesis. Researchers are building genetic modules that can control cell shape, direct pattern formation, or trigger multicellular assembly. The goal is to harness the rules of morphogenesis for applications in medicine, materials science, and biotechnology. This engineering approach tests our understanding of morphobioscience in the most rigorous way possible: by trying to build with its principles.
Programming Cellular Behavior and Tissue Engineering
A major frontier is the engineering of synthetic morphogenesis, where cells are programmed to self-organize into specific, pre-determined structures. Inspired by natural developmental processes, scientists design genetic circuits that control cell adhesion, differentiation, and movement. This has direct implications for regenerative medicine and the creation of artificial tissues and organs.
For instance, researchers have created systems where engineered cells can form simple patterns like stripes or spots, mimicking the early stages of biological patterning. These are the first steps toward building complex, functional tissues from the ground up. This work validates Jacob's vision of biology as an informational science governed by programmable logic.
- Logic Gates in Cells: Scientists implant synthetic versions of operons that function as AND, OR, and NOT gates, allowing for sophisticated decision-making within living cells.
- Pattern Formation: By engineering gradients of signaling molecules and responsive genetic circuits, researchers can guide cells to form spatial patterns, a foundational step in morphogenesis.
- Biofabrication: Programmed cells can be used as living factories to deposit specific materials, potentially growing structures like bone or cartilage in precise shapes.
Ethical and Philosophical Implications of Morphobioscience
The ability to understand and manipulate the fundamental processes of form raises significant ethical and philosophical questions. As morphobioscience progresses from explaining to engineering, it forces a re-examination of concepts like naturalness, identity, and the boundaries of life. The power to direct morphological outcomes carries with it a responsibility to consider long-term consequences.
Jacob himself was deeply reflective about the nature of life and scientific inquiry. His later writings grappled with the implications of seeing living systems as evolved historical objects and as complex machines. This dual perspective is central to modern debates in bioethics surrounding genetic modification, human enhancement, and synthetic life.
"What we can do, and what we ought to do, are separated by a chasm that science alone cannot bridge." - A reflection on the ethical dimension of biological engineering.
Reconciling Mechanism and Organicism
A persistent philosophical tension in biology is between mechanistic and organicist views of life. Jacob's "genetic program" metaphor leaned mechanistic, portraying the organism as executing coded instructions. Modern morphobioscience, with its emphasis on emergent properties and self-organization, reintroduces organicist principles.
The field today seeks a synthesis: organisms are mechanistic in their parts but organicist in their whole. They are built from molecular machines and genetic circuits, yet their final form arises from complex, dynamic interactions that are not fully predictable from parts alone. This synthesis provides a more complete and humble understanding of biological complexity.
This perspective cautions against reductionist overreach. While we can manipulate genes to influence form, the outcome is never guaranteed due to the network's robustness and adaptability. This inherent unpredictability is a crucial factor in ethical considerations about modifying complex biological systems.
Conclusion: The Integrated Narrative of Form and Information
The odyssey from François Jacob's discovery of the operon to the modern science of morphobioscience reveals an integrated narrative. It is the story of how biology learned to speak the language of information and control. Jacob's work provided the grammar—the rules of genetic regulation—that allowed scientists to begin reading the story of how form is written and rewritten through evolution.
Morphobioscience has expanded this narrative by adding the crucial chapters of physical forces, evolutionary history, and self-organization. It shows that the blueprint is not enough; you must also understand the materials, the environmental context, and the historical contingencies that guide construction. The field stands as a testament to the power of interdisciplinary synthesis in science.
Key Takeaways from Jacob's Legacy and Morphobioscience
- Genetic Regulation is Foundational: The operon model was a paradigm shift, revealing that genes are organized into regulated circuits, a principle governing all life.
- Evolution is a Tinkerer: New biological forms arise primarily from the modification and repurposing of existing genetic modules and developmental pathways.
- Form is an Emergent Property: Morphology results from the dynamic interplay between genetic information and physical processes within a three-dimensional environment.
- The Past Informs the Present: Understanding the history of an organism's lineage is essential to explaining its current form, as evolution works on inherited templates.
- Synthesis is the Future: The greatest insights will come from integrating genetics, development, evolution, and biophysics into a unified science of biological form.
The journey of scientific discovery chronicled here is far from over. The next chapters in morphobioscience will likely be written at the frontiers of computational prediction and synthetic construction. As we build increasingly accurate models and engineer more complex biological forms, we will continue to test and refine the principles first illuminated by pioneers like François Jacob.
The ultimate lesson is one of profound interconnection. The logic of life unveiled in a bacterial cell can inform our understanding of our own development and our place in the history of life on Earth. By continuing to explore the revelation and narrativization of these principles, science moves closer to a complete story—one that weaves together the threads of information, form, and time into a coherent understanding of the living world.
Max Delbrück: Nobel-Winning Pioneer of Molecular Biology
Introduction to a Scientific Revolutionary
Max Delbrück was a visionary scientist whose groundbreaking work in bacteriophage research laid the foundation for modern molecular biology. Born in Germany in 1906, Delbrück transitioned from physics to biology, forever changing our understanding of genetic structure and viral replication. His contributions earned him the 1969 Nobel Prize in Physiology or Medicine, shared with Salvador Luria and Alfred Hershey.
Early Life and Academic Foundations
Delbrück was born on September 4, 1906, in Berlin, Germany, into an academic family. His father, Hans Delbrück, was a prominent historian, while his mother came from a family of scholars. This intellectual environment nurtured young Max's curiosity and love for science.
Education and Shift from Physics to Biology
Delbrück initially pursued theoretical physics, earning his PhD from the University of Göttingen in 1930. His early work included a stint as an assistant to Lise Meitner in Berlin, where he contributed to the prediction of Delbrück scattering, a phenomenon involving gamma ray interactions.
Inspired by Niels Bohr's ideas on complementarity, Delbrück began to question whether similar principles could apply to biology. This curiosity led him to shift his focus from physics to genetics, a move that would redefine scientific research.
Fleeing Nazi Germany and Building a New Life
The rise of the Nazi regime in Germany forced Delbrück to leave his homeland in 1937. He relocated to the United States, where he continued his research at Caltech and later at Vanderbilt University. In 1945, he became a U.S. citizen, solidifying his commitment to his new home.
Key Collaborations and the Phage Group
Delbrück's most influential work began with his collaboration with Salvador Luria and Alfred Hershey. Together, they formed the Phage Group, a collective of scientists dedicated to studying bacteriophages—viruses that infect bacteria. Their research transformed phage studies into an exact science, enabling precise genetic investigations.
One of their most notable achievements was the development of the one-step bacteriophage growth curve in 1939. This method allowed researchers to track the replication cycle of phages, revealing that a single phage could produce hundreds of thousands of progeny within an hour.
Groundbreaking Discoveries in Genetic Research
Delbrück's work with Luria and Hershey led to several pivotal discoveries that shaped modern genetics. Their research provided critical insights into viral replication and the nature of genetic mutations.
The Fluctuation Test and Spontaneous Mutations
In 1943, Delbrück and Luria conducted the Fluctuation Test, a groundbreaking experiment that demonstrated the random nature of bacterial mutations. Their findings disproved the prevailing idea that mutations were adaptive responses to environmental stress. Instead, they showed that mutations occur spontaneously, regardless of external conditions.
This discovery was pivotal in understanding genetic stability and laid the groundwork for future studies on mutation rates and their implications for evolution.
Viral Genetic Recombination
In 1946, Delbrück and Hershey made another significant breakthrough by discovering genetic recombination in viruses. Their work revealed that viruses could exchange genetic material, a process fundamental to genetic diversity and evolution. This finding further solidified the role of phages as model organisms in genetic research.
Legacy and Impact on Modern Science
Delbrück's contributions extended beyond his immediate discoveries. His interdisciplinary approach, combining physics and biology, inspired a new generation of scientists. The Phage Group he co-founded became a training ground for many leaders in molecular biology, influencing research for decades.
The Nobel Prize and Beyond
In 1969, Delbrück was awarded the Nobel Prize in Physiology or Medicine for his work on viral replication and genetic structure. The prize recognized his role in transforming phage research into a precise scientific discipline, enabling advancements in genetics and molecular biology.
Even after receiving the Nobel Prize, Delbrück continued to push the boundaries of science. He challenged existing theories, such as the semi-conservative replication of DNA, and explored new areas like sensory transduction in Phycomyces, a type of fungus.
Conclusion of Part 1
Max Delbrück's journey from physics to biology exemplifies the power of interdisciplinary thinking. His work with bacteriophages not only advanced our understanding of genetics but also set the stage for modern molecular biology. In the next section, we will delve deeper into his later research, his influence on contemporary science, and the enduring legacy of his contributions.
Later Research and Challenging Established Theories
After receiving the Nobel Prize, Max Delbrück continued to push scientific boundaries through innovative experiments and theoretical challenges. His work remained focused on uncovering fundamental biological principles, often questioning prevailing assumptions.
Challenging DNA Replication Models
In 1954, Delbrück proposed a dispersive theory of DNA replication, challenging the dominant semi-conservative model. Though later disproven by Meselson and Stahl, his hypothesis stimulated critical debate and refined experimental approaches in molecular genetics.
Delbrück emphasized the importance of precise measurement standards, stating:
"The only way to understand life is to measure it as carefully as possible."This philosophy driven his entire career.
Studying Phycomyces Sensory Mechanisms
From the 1950s onward, Delbrück explored Phycomyces, a fungus capable of complex light and gravity responses. His research revealed how simple organisms translate environmental signals into measurable physical changes, bridging genetics and physiology.
- Demonstrated photoreceptor systems in fungal growth patterns
- Established quantitative methods for studying sensory transduction
- Influenced modern research on signal transduction pathways
The Max Delbrück Center: A Living Legacy
Following Delbrück's death in 1981, the Max Delbrück Center (MDC) was established in Berlin in 1992, embodying his vision of interdisciplinary molecular medicine. Today, it remains a global leader in genomics and systems biology.
Research Impact and Modern Applications
Delbrück's phage methodologies continue to underpin contemporary genetic technologies:
- CRISPR-Cas9 development builds on his quantitative phage genetics
- Modern viral vector engineering relies on principles he established
- Bacterial gene expression studies trace back to his fluctuation test designs
The MDC currently hosts over 1,500 researchers from more than 60 countries, continuing Delbrück's commitment to collaborative science.
Enduring Influence on Modern Genetics
Delbrück's approach to science—combining rigor, creativity, and simplicity—shapes current research paradigms. His emphasis on quantitative analysis remains central to modern genetic studies.
Philosophical Contributions
Delbrück advocated for studying biological systems at their simplest levels before tackling complexity. This "simplicity behind complexity" principle now guides systems biology and synthetic biology efforts worldwide.
His legacy endures through:
- Training generations of molecular biologists through the Phage Group
- Establishing foundational methods for mutant strain analysis
- Promoting international collaboration in life sciences
Legacy in Education and Mentorship
Max Delbrück’s influence extended far beyond his publications through his role as a mentor and educator. His leadership of the Phage Group created a model for collaborative, interdisciplinary training that shaped generations of scientists.
Training Future Scientists
Delbrück emphasized quantitative rigor and intellectual curiosity in his students. At Cold Spring Harbor, he fostered a community where physicists, biologists, and chemists worked together—a precursor to modern systems biology.
- Mentored Gordon Wolstenholme, who later directed the Salk Institute
- Inspired Walter Gilbert, a future Nobel laureate in chemistry
- Established a culture of critical debate that accelerated scientific progress
Current Applications of Delbrück's Work
Delbrück’s methods and discoveries remain embedded in today’s most advanced genetic technologies. His approach continues to inform cutting-edge research across multiple fields.
Impact on Modern Genetic Engineering
The principles Delbrück established through bacteriophage studies are foundational to tools transforming medicine and agriculture:
- CRISPR-Cas9 gene editing relies on phage-derived mechanisms
- Viral gene therapy vectors use designs first explored in his labs
- Bacterial mutagenesis studies follow protocols he refined
"Delbrück taught us to see genes not as abstract concepts, but as measurable molecular machines."
Advancing Genomics and Virology
Today’s genomic research owes a debt to Delbrück’s emphasis on precise measurement. Modern sequencing technologies and viral dating methods build directly on his frameworks.
Key ongoing applications include:
- Pandemic preparedness through phage-based virus tracking
- Cancer genomics using mutation rate analysis he pioneered
- Synthetic biology circuits inspired by his Phycomyces studies
Conclusion: The Enduring Impact of Max Delbrück
Max Delbrück transformed our understanding of life at the molecular level through visionary experiments, interdisciplinary collaboration, and unwavering intellectual rigor. His work remains a cornerstone of modern genetics.
Key Takeaways
The legacy of Delbrück endures through:
- Nobel-recognized discoveries in viral replication and mutation
- The Max Delbrück Center’s ongoing research in molecular medicine
- A scientific philosophy that values simplicity behind complexity
As biology grows increasingly complex, Delbrück’s insistence on quantitative clarity and collaborative inquiry continues to guide researchers worldwide. His life’s work proves that understanding life’s simplest mechanisms remains the surest path to unlocking its deepest mysteries.


Gregor Johann Mendel: The Father of Genetics Explained
Introduction to Gregor Johann Mendel
Gregor Johann Mendel, often referred to as the father of genetics, revolutionized our understanding of heredity through his meticulous experiments on pea plants. His groundbreaking work laid the foundation for modern genetics, introducing key principles such as dominant and recessive traits, segregation, and independent assortment. Despite the lack of verifiable information on the term "Grhgorios-Iwannhs-Mentel-O-Pateras-ths-Genetikhs," it is clear that Mendel's contributions are the cornerstone of genetic studies.
The Life and Work of Gregor Johann Mendel
Early Life and Background
Born in 1822 in what is now the Czech Republic, Mendel was an Augustinian friar with a deep interest in science. His early education in philosophy and natural sciences at the University of Olomouc and the University of Vienna equipped him with the knowledge to conduct his famous experiments.
Pioneering Experiments on Pea Plants
Between 1856 and 1863, Mendel conducted a series of experiments on pea plants, focusing on seven distinct traits. His quantitative approach allowed him to observe patterns of inheritance that had previously been misunderstood. By cross-breeding pea plants with different characteristics, Mendel was able to establish the principles of dominant and recessive traits.
Publication and Recognition
Mendel's findings were published in 1866 under the title "Experiments on Plant Hybridization." Although his work was initially overlooked, it was rediscovered in 1900, sparking a renewed interest in the field of genetics. This rediscovery marked the beginning of modern genetic research and solidified Mendel's place as the father of genetics.
Key Principles of Mendelian Genetics
Dominant and Recessive Traits
One of Mendel's most significant contributions was the identification of dominant and recessive traits. He observed that certain traits, such as flower color, would consistently appear in offspring, while others would seem to disappear, only to reappear in subsequent generations. This led to the understanding that traits are inherited through discrete units, now known as genes.
Principle of Segregation
The principle of segregation states that each individual possesses two alleles for each trait, one inherited from each parent. During the formation of gametes, these alleles segregate, or separate, so that each gamete carries only one allele for each trait. This principle explains the variation observed in offspring.
Principle of Independent Assortment
Mendel also discovered that different traits are inherited independently of one another. This principle of independent assortment means that the inheritance of one trait does not influence the inheritance of another. This finding was crucial for understanding the complexity of genetic inheritance.
Impact of Mendel's Work on Modern Genetics
Influence on Evolutionary Biology
Mendel's work had a profound impact on evolutionary biology. His principles provided a mechanism for understanding how traits are passed down through generations, which was essential for Charles Darwin's theory of natural selection. The integration of Mendelian genetics with evolutionary theory led to the development of the modern synthesis, a unified theory of evolution.
Foundation for DNA-Based Genetics
The principles established by Mendel laid the groundwork for the discovery of DNA and the field of molecular genetics. His work on inheritance patterns provided the framework for understanding how genes are transmitted and expressed, which was later expanded upon with the discovery of the structure of DNA by James Watson and Francis Crick.
Applications in Modern Science
Today, Mendel's principles are applied in various fields, including agriculture, medicine, and biotechnology. Genetic engineering, gene therapy, and the study of genetic disorders all owe their foundations to Mendel's pioneering work. His contributions continue to influence scientific research and technological advancements.
Common Misconceptions and Clarifications
Addressing the Term "Grhgorios-Iwannhs-Mentel-O-Pateras-ths-Genetikhs"
The term "Grhgorios-Iwannhs-Mentel-O-Pateras-ths-Genetikhs" appears to be a blend of Greek and a garbled form of Gregor Johann Mendel. There is no verifiable information on this term as a specific person, entity, or established topic in genetics. It is likely a misspelling or pseudonym, and the intended reference is Gregor Johann Mendel, the actual father of genetics.
Clarifying Mendel's Role in Genetics
While Mendel's work is foundational, it is important to note that modern genetics has evolved significantly since his time. Advances in technology and our understanding of DNA have expanded upon Mendel's principles, providing a more comprehensive view of genetic inheritance and variation.
Conclusion
Gregor Johann Mendel's contributions to the field of genetics are immeasurable. His pioneering experiments on pea plants established the core principles of inheritance, laying the foundation for modern genetics. Despite the lack of information on the term "Grhgorios-Iwannhs-Mentel-O-Pateras-ths-Genetikhs," it is clear that Mendel's work remains a cornerstone of genetic research. His legacy continues to influence scientific advancements and our understanding of the natural world.
Mendel's Legacy in Modern Genetic Research
From Pea Plants to Human Genetics
Mendel's work on pea plants may seem simple by today's standards, but his principles have been applied to complex human genetic studies. Researchers now use genome-wide association studies (GWAS) to identify genetic variants linked to diseases, building on Mendel's foundational ideas of trait inheritance. His methods of controlled experimentation and quantitative analysis remain essential in genetic research.
Advancements in Genetic Technology
The development of CRISPR gene editing and other genetic technologies can trace their roots back to Mendel's discoveries. These tools allow scientists to manipulate genes with precision, opening new possibilities for treating genetic disorders. Mendel's emphasis on systematic observation and data collection continues to guide modern geneticists.
Mendelian Genetics in Agriculture
Selective Breeding and Crop Improvement
Mendel's principles have had a profound impact on agriculture. Through selective breeding, farmers and scientists have developed crops with desirable traits such as disease resistance, higher yields, and improved nutritional content. This process relies on Mendel's understanding of dominant and recessive traits to achieve consistent results.
Genetically Modified Organisms (GMOs)
The creation of genetically modified organisms (GMOs) is another application of Mendelian genetics. By introducing specific genes into crops, scientists can enhance their resistance to pests, tolerance to environmental stress, and overall productivity. These advancements have significantly contributed to global food security.
Challenges and Controversies in Mendelian Genetics
Limitations of Mendel's Principles
While Mendel's principles are foundational, they do not account for all aspects of genetic inheritance. Polygenic traits, which are influenced by multiple genes, and epigenetics, which involves changes in gene expression without altering the DNA sequence, present complexities that Mendel's simple models do not address. These limitations highlight the need for ongoing research and refinement of genetic theories.
Ethical Considerations in Genetic Research
The application of Mendelian genetics in modern science raises important ethical questions. Issues such as genetic privacy, the potential for designer babies, and the equitable distribution of genetic technologies are subjects of ongoing debate. Mendel's work, while groundbreaking, also serves as a reminder of the responsibility that comes with scientific advancement.
Recent Developments in Genetics
Paternal Age and Genetic Disorders
Recent studies have explored the impact of paternal age on genetic disorders. Research indicates that de novo mutations in sperm increase with age, contributing to conditions such as autism and schizophrenia. A study published in Nature found that while these mutations confer a small risk, they do not fully explain the epidemiologic links observed in Danish registries.
Population Genetics and Ancestry
Advances in population genetics have revealed fascinating insights into human ancestry. For example, studies on the Iberian Roma have shown that Y-chromosome haplogroups H and J2a1b are dominant, indicating a strong paternal South Asian ancestry. This research underscores the complexity of genetic inheritance and the influence of historical migrations.
Genetic Mosaicism and Disease
Genetic mosaicism, where an individual has more than one genetic lineage, has been a focus of recent research. A study on NLRP3 variants found that 16 out of 17 cases with CAPS (Cryopyrin-Associated Periodic Syndromes) phenotypes exhibited mosaicism. This highlights the importance of understanding genetic variation in diagnosing and treating genetic disorders.
Mendel's Influence on Education and Public Understanding
Teaching Mendelian Genetics
Mendel's principles are a cornerstone of biology education. Students worldwide learn about Punnett squares and Mendelian inheritance as part of their basic genetic education. These tools provide a simple yet powerful way to predict the outcomes of genetic crosses, making complex concepts accessible to learners of all levels.
Public Perception and Misconceptions
Despite the widespread teaching of Mendelian genetics, there are common misconceptions. Many people believe that traits are solely determined by a single gene, ignoring the influence of environmental factors and polygenic inheritance. Educators and scientists continue to work on clarifying these misunderstandings to promote a more accurate understanding of genetics.
Future Directions in Genetic Research
Personalized Medicine
The future of genetics lies in personalized medicine, where treatments are tailored to an individual's genetic makeup. This approach promises to revolutionize healthcare by providing more effective and targeted therapies. Mendel's principles of inheritance are fundamental to understanding the genetic basis of diseases and developing personalized treatment plans.
Genetic Research and Global Health
Genetic research has the potential to address global health challenges. By studying the genetic basis of diseases, scientists can develop vaccines, treatments, and preventive measures that are more effective and accessible. Mendel's legacy continues to inspire researchers to explore the genetic underpinnings of health and disease, ultimately improving the quality of life for people worldwide.
Conclusion
Gregor Johann Mendel's contributions to genetics have had a lasting impact on science, agriculture, and medicine. His principles of inheritance remain fundamental to our understanding of genetics, and his work continues to inspire new discoveries and advancements. As we look to the future, Mendel's legacy serves as a reminder of the power of systematic observation, experimentation, and the pursuit of knowledge.
The Enduring Impact of Mendel's Work on Scientific Thought
Integration with Evolutionary Theory
Mendel's principles provided the missing link in Charles Darwin's theory of evolution. While Darwin proposed natural selection as the mechanism for evolution, he lacked an explanation for how traits were inherited. Mendel's discovery of discrete hereditary units (genes) and their predictable patterns of transmission filled this gap, leading to the modern synthesis of evolutionary biology in the early 20th century.
Quantitative Genetics and Beyond
The mathematical foundation of Mendel's work paved the way for quantitative genetics, which studies traits influenced by multiple genes. This field has been crucial in understanding complex characteristics such as height, intelligence, and susceptibility to diseases. Mendel's emphasis on statistical analysis remains a cornerstone of genetic research, enabling scientists to model and predict genetic outcomes with greater accuracy.
Mendel's Influence on Related Scientific Disciplines
Molecular Biology and the Discovery of DNA
Mendel's work set the stage for the discovery of DNA as the hereditary material. Scientists like James Watson and Francis Crick built upon Mendelian principles to unravel the structure of DNA in 1953. This breakthrough confirmed the physical basis of Mendel's abstract hereditary factors, revolutionizing our understanding of how genetic information is stored and transmitted.
Genomics and Bioinformatics
The field of genomics, which involves the study of entire genomes, owes much to Mendel's foundational work. Modern bioinformatics tools use Mendelian principles to analyze vast amounts of genetic data, identifying gene functions and interactions. Techniques such as genome-wide association studies (GWAS) rely on the concepts of genetic linkage and inheritance that Mendel first described.
Practical Applications of Mendelian Genetics
Medical Genetics and Disease Prevention
Mendel's principles are applied in medical genetics to understand and predict the inheritance of genetic disorders. Conditions such as cystic fibrosis, sickle cell anemia, and Huntington's disease follow Mendelian inheritance patterns, allowing for genetic counseling and predictive testing. Early identification of carriers and affected individuals can lead to better management and prevention strategies.
- Autosomal dominant disorders (e.g., Huntington's disease) require only one copy of the mutated gene.
- Autosomal recessive disorders (e.g., cystic fibrosis) require two copies of the mutated gene.
- X-linked disorders (e.g., hemophilia) are carried on the X chromosome and affect males more frequently.
Agricultural Advancements and Food Security
The application of Mendelian genetics in agriculture has led to significant improvements in crop and livestock breeding. Through selective breeding and hybridization, scientists and farmers have developed varieties with desirable traits such as disease resistance, drought tolerance, and enhanced nutritional value. These advancements are critical in addressing global food security challenges.
"Mendel's work on pea plants demonstrated that genetic traits could be predicted and manipulated, a principle that underpins all modern agricultural biotechnology."
Ethical and Social Implications of Mendelian Genetics
Genetic Testing and Privacy Concerns
The ability to predict genetic traits and disorders raises important ethical considerations. Genetic testing can provide valuable information about an individual's health risks, but it also poses challenges related to privacy and discrimination. Laws such as the Genetic Information Nondiscrimination Act (GINA) in the United States aim to protect individuals from genetic discrimination in employment and health insurance.
The Debate Over Genetic Engineering
Advancements in genetic engineering, such as CRISPR-Cas9, have sparked debates about the ethical boundaries of manipulating genetic material. While these technologies hold promise for treating genetic disorders and improving crop yields, they also raise concerns about unintended consequences and the potential for "designer babies." Mendel's work, while foundational, serves as a reminder of the need for responsible and ethical application of genetic knowledge.
Challenges and Future Prospects in Genetics
Addressing Complex Genetic Disorders
Many genetic disorders do not follow simple Mendelian patterns and are influenced by multiple genes and environmental factors. Conditions such as heart disease, diabetes, and certain cancers present significant challenges due to their polygenic nature. Future research aims to unravel these complexities, leveraging advanced technologies and interdisciplinary approaches.
The Role of Epigenetics
Epigenetics, the study of heritable changes in gene expression that do not involve alterations to the underlying DNA sequence, is an emerging field that complements Mendelian genetics. Understanding how environmental factors and lifestyle choices can influence gene expression offers new avenues for disease prevention and treatment. This area of research highlights the dynamic interplay between genetics and the environment.
Educational Initiatives and Public Engagement
Promoting Genetic Literacy
Efforts to improve genetic literacy are essential for empowering individuals to make informed decisions about their health and the health of future generations. Educational programs and public outreach initiatives aim to demystify genetic concepts, making them accessible to non-scientists. Understanding basic genetic principles can help people navigate genetic testing, family planning, and personalized medicine.
Museums and Historical Recognition
Mendel's contributions are celebrated in museums and educational institutions worldwide. The Mendel Museum in Brno, Czech Republic, honors his life and work, offering exhibits that explore his experiments and their impact on modern science. Such initiatives ensure that Mendel's legacy continues to inspire future generations of scientists and researchers.
Conclusion: The Lasting Legacy of Gregor Johann Mendel
Gregor Johann Mendel's groundbreaking work on inheritance has left an indelible mark on the field of genetics and beyond. His principles of dominant and recessive traits, segregation, and independent assortment remain fundamental to our understanding of genetic inheritance. From advancing medical genetics to revolutionizing agriculture, Mendel's contributions have shaped numerous scientific disciplines and practical applications.
As we continue to explore the complexities of the genetic world, Mendel's legacy serves as a reminder of the power of curiosity, systematic observation, and rigorous experimentation. His work not only laid the foundation for modern genetics but also demonstrated the importance of interdisciplinary collaboration and ethical consideration in scientific advancement.
In an era of rapid technological progress and genetic innovation, Mendel's principles continue to guide researchers and practitioners. The future of genetics holds immense promise, from personalized medicine to sustainable agriculture, all rooted in the foundational discoveries of the father of genetics. As we stand on the shoulders of this scientific giant, we are reminded of the enduring impact of one man's curiosity and the pea plants that changed the world.
Demystifying Zak-Mono-O-8rylos-ths-Moriakhs-Biologias
The search for a clear definition of Zak-Mono-O-8rylos-ths-Moriakhs-Biologias presents a unique puzzle. A thorough review of modern scientific literature reveals no direct matches for this term in biological nomenclature or established fields. This analysis will decode the probable origins of this phrase, connecting its components to real and evolving concepts in modern biology. Our journey begins by examining the historical and contemporary scientific ideas it appears to reference.
This investigation into Zak-Mono-O-8rylos-ths-Moriakhs-Biologias underscores the importance of precise terminology in science. The term seems to be a composite, blending elements from biological classification, microbiological study, and advanced analytical techniques. Understanding its potential meaning requires exploring the significant shifts in how life is categorized and studied today.
Decoding the Term: Historical Roots and Modern Science
The phrase Zak-Mono-O-8rylos-ths-Moriakhs-Biologias appears to intertwine several biological concepts. The most recognizable element is "Mono," which likely references the historical kingdom Monera. This kingdom was used for decades to classify prokaryotic organisms—those without a cell nucleus, such as bacteria. However, major advancements in phylogenetic analysis have rendered this classification obsolete.
The other components, "O-8rylos" and "Moriakhs Biologias," suggest connections to modern omics technologies and microbiological research. By breaking down each part, we can trace a path from outdated taxonomy to the cutting-edge, data-driven biology of the 21st century. This reflects the dynamic and self-correcting nature of scientific progress.
The Legacy and Evolution of Monera
The term Monera originates from the Greek word for "solitary," describing simple, single-celled life forms. For much of the 20th century, it served as one of the five kingdoms in a widely used biological classification system. This kingdom grouped all prokaryotes together, primarily bacteria and what were then called "blue-green algae."
This classification was fundamentally challenged by the work of Carl Woese in 1977. By comparing ribosomal RNA sequences, Woese revealed a deep evolutionary split among prokaryotes. This led to the revolutionary three-domain system of life: Bacteria, Archaea, and Eukarya. Consequently, the kingdom Monera was abandoned as it represented a paraphyletic group, not a true evolutionary lineage.
- Monera is an outdated taxonomic category.
- Modern classification uses the domains Bacteria and Archaea.
- This change was driven by genetic evidence, showcasing the power of molecular biology.
Connections to Omics and Microbiological Research
The "O-8rylos" component bears a phonetic resemblance to the suffix "-omics," which is central to contemporary biology. Omics refers to the collective technologies used to explore the roles, relationships, and actions of various types of molecules that make up a cell. This field represents a paradigm shift from studying single genes or proteins to analyzing entire systems.
The integration of omics technologies has been described as the cornerstone of 21st-century systems biology, enabling holistic views of biological functions.
Similarly, "Moriakhs Biologias" seems to relate to microbiology, the study of microorganisms. Today, microbiology is inextricably linked with omics approaches. The field of microbiomics, for example, uses genomic techniques to study entire microbial communities, known as microbiomes, in environments ranging from the human gut to ocean floors.
Why the Term Appears Fabricated in Modern Literature
Extensive searches across authoritative databases yield no results for Zak-Mono-O-8rylos-ths-Moriakhs-Biologias as a legitimate scientific term. This includes checks of taxonomic authorities like the NCBI (National Center for Biotechnology Information), scientific publication indexes like PubMed and Google Scholar, and standard biological references. This absence is a key piece of evidence.
The lack of credible sources suggests the term may be a garbled or constructed reference. It potentially combines a misspelling or variant of "Monera" with stylized versions of "omics" and "microbiology." In the digital age, such strings can sometimes arise from translation errors, speculative fiction, or even AI-generated text. It serves as a reminder of the critical need for source verification in scientific journalism.
The Critical Role of Accurate Nomenclature
Precise and standardized language is the bedrock of scientific communication. A term like Zak-Mono-O-8rylos-ths-Moriakhs-Biologias, which has no established definition, creates confusion and hinders knowledge sharing. Scientific progress depends on researchers worldwide having a common, unambiguous vocabulary.
Established terms like microbiology, genomics, and prokaryote carry specific meanings backed by decades of peer-reviewed research. They allow for accurate hypothesis testing, replication of experiments, and effective education. The evolution from Monera to Bacteria and Archaea is a prime example of nomenclature refining itself to reflect better evidence.
The Real Scientific Trends Underlying the Phrase
While the specific term is not recognized, the concepts it points toward are at the forefront of modern biology. The intersection of microbiology, high-throughput omics technologies, and computational analysis defines today's most exciting research avenues. These fields are solving complex problems in health, environment, and industry.
For instance, metabolomics (an omics field) profiles the small-molecule metabolites in a cell, providing a snapshot of its physiological state. When applied to microbiomes, it can reveal how gut bacteria affect human health. Furthermore, tools like AlphaFold have revolutionized structural biology by predicting protein folds with high accuracy, solving a 50-year-old grand challenge.
- Omics integration is driving breakthroughs in personalized medicine and environmental science.
- AI and machine learning are now indispensable for analyzing complex biological data sets.
- Modern microbiology focuses on community interactions (microbiomes) rather than isolated species.
These real-world trends highlight the vast distance between a nonsensical term and the rigorous, impactful science being conducted globally. The next part of this analysis will delve deeper into these contemporary fields, exploring their methods, applications, and future directions.
The Ascendancy of Omics Technologies in Modern Biology
The suffix "-omics" has become ubiquitous in life sciences, representing a fundamental shift toward large-scale data analysis. These technologies enable scientists to study biological systems holistically rather than one component at a time. The field has expanded dramatically since the completion of the Human Genome Project, moving from genomics to proteomics, metabolomics, and beyond.
This data-driven approach is essential for understanding complex biological networks. By analyzing complete sets of molecules, researchers can identify patterns and interactions that were previously invisible. This holistic view is crucial for tackling multifaceted challenges in medicine, agriculture, and environmental science.
Key Omics Disciplines and Their Impact
Genomics was the pioneer, focusing on the complete set of DNA within an organism. It has paved the way for personalized medicine, where treatments can be tailored to an individual's genetic makeup. The cost of sequencing a human genome has plummeted from billions of dollars to under $1,000, making it accessible for widespread research and clinical use.
Proteomics studies the entire set of proteins expressed by a genome. Proteins are the workhorses of the cell, and understanding their structures and functions is vital. Advances like AlphaFold's AI-powered protein structure prediction have dramatically accelerated this field, solving structures in minutes that once took years.
- Transcriptomics: Analyzes all RNA molecules to understand gene expression patterns.
- Metabolomics: Focuses on the complete set of small-molecule metabolites, providing a snapshot of cellular physiology.
- Microbiomics: Studies the collective genomes of microbial communities, revolutionizing our understanding of health and disease.
The Convergence of Omics and Data Science
The sheer volume of data generated by omics technologies necessitates sophisticated computational tools. Bioinformatics has emerged as a critical discipline, blending biology, computer science, and information technology. Researchers use machine learning algorithms to sift through massive datasets, identifying meaningful biological signals from noise.
It is estimated that the total volume of biological data is doubling approximately every 18 months, highlighting the critical need for advanced computational infrastructure.
This convergence is leading to new discoveries about the interconnectedness of biological systems. For example, integrating genomic, proteomic, and metabolomic data can reveal how a genetic mutation affects protein function and ultimately alters cellular metabolism. This systems biology approach is the true legacy of the omics revolution.
Microbiology's Transformation in the 21st Century
Modern microbiology has moved far beyond the simple observation of organisms under a microscope. The field is now defined by its integration with molecular biology and omics technologies. This has transformed our understanding of microbes from isolated pathogens to integral components of complex ecosystems.
The concept of the holobiont—a host and its entire microbial community—has become a central theme. Researchers now recognize that human health, plant vitality, and environmental balance are deeply influenced by these dynamic partnerships. This shift represents a paradigm change in biological thinking.
The Human Microbiome: A Frontier for Health
The human body is home to trillions of microorganisms, collectively known as the human microbiome. These microbes play essential roles in digestion, immunity, and even mental health. The National Institutes of Health's Human Microbiome Project has been instrumental in cataloging these communities and understanding their functions.
Dysbiosis, or an imbalance in the microbiome, is linked to a wide range of conditions. These include inflammatory bowel disease, obesity, allergies, and neurological disorders. Therapies like fecal microbiota transplants have shown remarkable success in treating recurrent C. difficile infections, demonstrating the therapeutic potential of manipulating the microbiome.
- The human gut microbiome alone can contain over 1,000 different bacterial species.
- Microbes in the human body outnumber human cells by an estimated ratio of 1.3 to 1.
- The total genetic material of the microbiome (the microbiome) is 100 times larger than the human genome.
Environmental and Industrial Applications
Beyond human health, microbiology is critical for addressing environmental challenges. Bioremediation uses microorganisms to degrade environmental pollutants like oil spills and industrial waste. Specific bacterial strains can break down toxic compounds into harmless substances, offering a natural cleanup solution.
In industry, microbes are engineered to produce biofuels, pharmaceuticals, and enzymes. This field, known as industrial microbiology or biotechnology, relies on genetic engineering and fermentation technology. The global market for microbial products is valued in the hundreds of billions of dollars, underscoring its economic importance.
The Tools Driving Biological Discovery Forward
The pace of discovery in biology is intrinsically linked to technological advancement. The development of new instruments and computational methods continually opens new frontiers for research. These tools allow scientists to ask questions that were previously impossible to answer.
From next-generation sequencers that read DNA at unprecedented speeds to cryo-electron microscopes that visualize molecules in atomic detail, technology is the engine of modern biology. The integration of artificial intelligence is the latest and perhaps most transformative wave of innovation.
Next-Generation Sequencing (NGS)
Next-Generation Sequencing technologies have democratized genomic analysis. They allow for the rapid and cost-effective sequencing of entire genomes or targeted regions of DNA and RNA. This has enabled large-scale population studies, cancer genomics, and real-time pathogen surveillance, as seen during the COVID-19 pandemic.
The data output from a single NGS run can be terabytes in size, necessitating robust data management and analysis pipelines. The continuous improvement of these platforms is pushing the limits of speed, accuracy, and affordability, making genomic medicine a reality.
The AI Revolution in Biology
Artificial intelligence, particularly deep learning, is reshaping biological research. AlphaFold's success in predicting protein structures is a landmark achievement. By accurately predicting the 3D structure of proteins from their amino acid sequences, AI is solving one of biology's longest-standing problems.
DeepMind's AlphaFold database has released predicted structures for over 200 million proteins, encompassing almost all known proteins sequenced to date.
AI applications now extend to drug discovery, where algorithms can predict the interaction between potential drug compounds and their targets. It is also used to analyze medical images, identify patterns in omics data, and model complex biological systems. The synergy between biology and AI is creating a new era of predictive and personalized science.
Continued advancements in these tools promise to further illuminate the complexities of life. The final section of this analysis will explore the ethical considerations and future directions shaped by these powerful technologies, cementing the vast gap between fabricated terminology and tangible scientific progress.
Ethical Considerations in the Age of Advanced Biology
The unprecedented power of modern biological tools brings profound ethical responsibilities. The ability to sequence genomes, engineer microbes, and manipulate biological systems demands careful ethical and societal scrutiny. Questions of privacy, equity, and safety must be addressed proactively by scientists, policymakers, and the public.
Issues like genetic discrimination, data ownership, and environmental release of engineered organisms are central to contemporary debates. The gap between technological capability and ethical frameworks highlights the need for ongoing dialogue. Responsible innovation requires balancing potential benefits with respect for individual rights and ecological stability.
Genomic Data Privacy and Security
As genomic sequencing becomes routine in healthcare and consumer services, protecting individual genetic data is paramount. This information is uniquely personal and sensitive, revealing predispositions to diseases, ancestry, and familial connections. Breaches of this data could lead to discrimination by employers or insurers.
Robust legal frameworks, such as the Genetic Information Nondiscrimination Act (GINA) in the United States, aim to prevent such misuse. However, laws often lag behind technology. Ensuring true informed consent and implementing state-of-the-art cybersecurity for genomic databases are ongoing challenges for the scientific community.
- Genomic data is fundamentally identifiable and cannot be fully anonymized.
- Over 80% of participants in large genomic studies can be identified using public data and simple tools.
- Clear policies on data sharing for research must balance privacy with scientific progress.
The Ethics of Gene Editing and Synthetic Biology
Technologies like CRISPR-Cas9 have made gene editing remarkably precise and accessible. While offering hope for curing genetic disorders, they also open the door to heritable human genome modifications. The 2018 case of gene-edited human babies in China sparked international condemnation and calls for a global moratorium on such applications.
The global scientific consensus strongly advises against the clinical use of heritable human genome editing until safety and ethical implications are fully resolved.
In synthetic biology, scientists can design and construct new biological parts and systems. This raises questions about biosecurity and the potential for creating harmful pathogens. A culture of responsible research and strong oversight institutions is essential to harness these technologies for public good while minimizing risks.
The Future Trajectory of Biological Science
Biology is evolving from a descriptive science to a predictive and engineering discipline. The convergence of biology with fields like computer science, engineering, and material science is creating entirely new possibilities. This interdisciplinary approach will define the next era of discovery and innovation.
Key trends include the move toward precision biology, where interventions are tailored to the individual's unique genetic and molecular profile. Furthermore, understanding complex ecosystems through integrated omics will be crucial for addressing climate change and biodiversity loss.
Personalized Medicine and Digital Health
The future of healthcare lies in personalized medicine, leveraging genomic, proteomic, and metabolomic data to customize prevention, diagnosis, and treatment. Cancer therapy is already being transformed by this approach, with treatments selected based on the specific genetic mutations of a patient's tumor.
Wearable devices and continuous health monitoring will generate real-time streams of biological data. Integrating this data with AI analysis will enable predictive health alerts and highly individualized wellness plans. This shift promises to move healthcare from a reactive to a proactive model.
Climate Change and Environmental Biology
Biology will play a central role in mitigating and adapting to climate change. Research focuses on developing carbon-capturing microbes, engineering drought-resistant crops, and protecting biodiversity through genomic conservation. Studying microbiomes in soil and oceans is key to understanding carbon and nutrient cycles.
Synthetic biology offers tools to create sustainable alternatives to petroleum-based products, such as biofuels and biodegradable plastics. These bio-based economies could significantly reduce humanity's environmental footprint. The application of biological solutions to global challenges is a major frontier for the coming decades.
Conclusion: From Fabricated Terms to Foundational Science
Our exploration of Zak-Mono-O-8rylos-ths-Moriakhs-Biologias has illuminated a critical point in scientific discourse: the importance of precise, evidence-based terminology. This fabricated term stands in stark contrast to the rigorous, dynamic, and transformative fields it inadvertently references—namely, the evolution from the outdated kingdom Monera to modern microbiology and omics-driven systems biology.
The journey from historical classification to cutting-edge research underscores science's self-correcting nature. Modern biology is not defined by obscure or nonsensical phrases but by concrete concepts, verifiable data, and powerful technologies that are reshaping our world.
Key Takeaways and Final Insights
The investigation reveals several core truths about contemporary biology. First, scientific progress is built on the foundation of clear communication and shared definitions. Second, fields like microbiomics, proteomics, and computational biology represent the real and impactful vanguard of life sciences.
Finally, the ethical integration of these powerful tools is as important as the discoveries themselves. The future of biology will be written not by fabricated terms, but by the responsible application of knowledge to improve human health, steward the environment, and deepen our understanding of life itself.
- Modern biology is interdisciplinary, data-rich, and ethically complex.
- Technological convergence (AI, omics, engineering) is accelerating the pace of discovery.
- Verification through authoritative sources remains the cornerstone of credible scientific information.
In closing, while Zak-Mono-O-8rylos-ths-Moriakhs-Biologias itself holds no scientific validity, the exercise of deconstructing it leads us directly to the heart of today's most exciting biological research. It serves as a reminder to critically evaluate sources and to appreciate the immense, collaborative effort required to advance our genuine understanding of the natural world. The true story of biology is far more compelling than any fabricated term could ever be.
Ernst Ruska: The Father of Electron Microscopy
Ernst Ruska, a pioneering German physicist, revolutionized the field of microscopy with his invention of the electron microscope. His groundbreaking work in the early 20th century laid the foundation for modern imaging technologies, enabling scientists to explore the microscopic world at unprecedented resolutions.
Early Life and Education
Born in 1906, Ernst Ruska showed an early aptitude for science and engineering. He pursued his studies at the Technical University of Munich and later at the Technische Hochschule Berlin, where he delved into high-voltage research and cathode-ray oscillograph calculations. His academic journey was marked by a keen interest in the behavior of electrons and their potential applications in imaging.
Academic Foundations
Ruska's early work was influenced by the theories of Hans Busch, who in 1926 proposed that magnetic fields could bundle electrons in a manner similar to how lenses focus light. This concept became a cornerstone of Ruska's later inventions. During his studies, he also collaborated with Max Knoll, a partnership that would prove instrumental in the development of the electron microscope.
The Invention of the Electron Microscope
The electron microscope was a monumental leap forward in imaging technology. Unlike traditional optical microscopes, which are limited by the wavelength of visible light, electron microscopes use beams of electrons to achieve far greater resolutions. This innovation allowed scientists to observe structures at the atomic level, opening new avenues in fields such as biology, materials science, and nanotechnology.
Key Milestones
On March 9, 1931, Ruska and Knoll achieved a significant breakthrough: the first two-stage electron-optical magnification using magnetic lenses. This milestone was built on Busch's earlier theories and marked the beginning of a new era in microscopy. By December 1933, Ruska's prototype had already surpassed the resolution capabilities of light microscopes, achieving a magnification of 12,000x.
- 1931: First two-stage electron-optical magnification
- 1933: Prototype exceeds light microscope resolution
- 1938–1939: First serial-production electron microscope developed at Siemens
Commercialization and Impact
With the assistance of Bodo von Borries, Ruska developed the first commercially viable electron microscope at Siemens. This instrument enabled atomic-scale imaging, revolutionizing scientific research and industrial applications. The ability to visualize structures at such minute scales had a profound impact on various disciplines, from biology to materials science.
Recognition and Legacy
Ernst Ruska's contributions to science were recognized with numerous accolades, culminating in the Nobel Prize in Physics in 1986. He shared this prestigious award with Gerd Binnig and Heinrich Rohrer for their work on scanning tunneling microscopy. Ruska's electron microscope, initially termed the "Übermikroskop," has left an indelible mark on the scientific community, spurring advancements in nanotechnology, virology, and beyond.
Preservation and Influence
The original electron microscope developed by Ruska is preserved at the Deutsches Museum in Munich, serving as a testament to his ingenuity. Modern electron microscopy continues to evolve, integrating high-performance computing and AI-enhanced image processing to achieve dynamic 3D reconstructions and sub-angstrom resolutions. Educational videos and resources from 2023 highlight the ongoing evolution of electron microscopy, from Ruska's early prototypes to advanced techniques like scanning electron microscopy (SEM) and transmission electron microscopy (TEM).
Technical Innovations and Advancements
The electron microscope operates on the principle of using electrons instead of light to illuminate specimens. This approach leverages the much shorter wavelength of electrons, approximately 100,000 times shorter than that of visible light, to achieve superior resolution. The electrons are focused using magnetic lenses, a concept derived from Busch's theories, and deflected by atoms within the specimen to create contrast.
Resolution and Magnification
The resolution capabilities of electron microscopes are truly remarkable. While traditional light microscopes are limited to resolutions of about 200 nanometers, electron microscopes can achieve resolutions as fine as 0.1 nanometers. This leap in resolution has enabled scientists to visualize structures at the atomic level, providing unprecedented insights into the fundamental building blocks of matter.
"The electron microscope has revolutionized our understanding of the microscopic world, enabling us to see what was previously invisible."
Early prototypes of the electron microscope achieved magnifications of up to 12,000x, a feat that was unthinkable with light microscopes. Modern electron microscopes can exceed magnifications of millions-fold, allowing for detailed observations of complex structures such as proteins, viruses, and nanomaterials.
Applications and Impact
The impact of the electron microscope extends across numerous scientific disciplines. In biology, it has enabled the visualization of cellular structures, viruses, and macromolecules, providing critical insights into biological processes. In materials science, electron microscopy has facilitated the study of crystalline structures, defects, and nanomaterials, driving advancements in technology and engineering.
- Biology: Visualization of cellular structures and macromolecules
- Materials Science: Study of crystalline structures and nanomaterials
- Nanotechnology: Exploration of atomic-scale structures and properties
The advent of techniques such as cryo-electron microscopy and aberration-corrected lenses has further expanded the capabilities of electron microscopy. These advancements have enabled the visualization of protein structures at sub-angstrom resolutions and the creation of dynamic 3D reconstructions, pushing the boundaries of scientific exploration.
Conclusion
Ernst Ruska's invention of the electron microscope has had a transformative impact on science and technology. His pioneering work has enabled researchers to explore the microscopic world at unprecedented levels of detail, driving advancements in fields ranging from biology to materials science. As electron microscopy continues to evolve, incorporating cutting-edge technologies such as AI and high-performance computing, Ruska's legacy remains a cornerstone of modern scientific discovery.
Ernst Ruska's Contributions to Modern Science
Ernst Ruska's groundbreaking work on the electron microscope not only revolutionized imaging technology but also had a profound impact on various scientific disciplines. His invention enabled researchers to explore the microscopic world with unprecedented clarity, leading to significant advancements in fields such as biology, materials science, and nanotechnology.
Advancements in Biology
The electron microscope has been instrumental in the field of biology, allowing scientists to visualize cellular structures, viruses, and macromolecules at the atomic level. This capability has provided critical insights into biological processes, enabling researchers to better understand the fundamental mechanisms of life.
- Cellular Structures: Detailed imaging of organelles and intracellular components
- Virology: Visualization of viral particles and their interactions with host cells
- Macromolecules: Study of complex biological molecules such as proteins and nucleic acids
One of the most significant contributions of electron microscopy to biology has been in the field of virology. The ability to visualize viral particles has been crucial in understanding viral structures, replication mechanisms, and interactions with host cells. This knowledge has been instrumental in the development of vaccines and antiviral therapies.
Impact on Materials Science
In the realm of materials science, the electron microscope has enabled researchers to study the properties and behaviors of materials at the atomic scale. This has led to the development of new materials with enhanced properties, as well as a deeper understanding of the fundamental principles governing material behavior.
- Crystalline Structures: Analysis of atomic arrangements and defects in crystals
- Nanomaterials: Exploration of the unique properties of materials at the nanoscale
- Material Properties: Investigation of mechanical, electrical, and thermal properties
The electron microscope has been particularly valuable in the study of nanomaterials. The ability to visualize and manipulate materials at the nanoscale has led to the development of novel materials with unique properties, such as enhanced strength, conductivity, and reactivity. These advancements have had a significant impact on industries ranging from electronics to medicine.
The Evolution of Electron Microscopy
Since the invention of the first electron microscope by Ernst Ruska and Max Knoll in 1931, the technology has undergone significant advancements. Modern electron microscopes incorporate cutting-edge technologies such as high-performance computing, AI-enhanced image processing, and advanced imaging techniques, enabling researchers to explore the microscopic world with unprecedented detail and precision.
From Static to Dynamic Imaging
Early electron microscopes were limited to static imaging, providing two-dimensional snapshots of specimens. However, modern electron microscopy has evolved to include dynamic imaging capabilities, allowing researchers to observe processes and interactions in real-time. This has been particularly valuable in the study of biological systems, where dynamic processes such as cellular interactions and molecular dynamics can be visualized.
- 3D Imaging: Reconstruction of three-dimensional structures from two-dimensional images
- Time-Resolved Imaging: Observation of processes and interactions in real-time
- Correlative Microscopy: Integration of multiple imaging techniques for comprehensive analysis
One of the most significant advancements in electron microscopy has been the development of 3D imaging techniques. By combining multiple two-dimensional images, researchers can reconstruct three-dimensional structures, providing a more comprehensive understanding of complex systems. This capability has been particularly valuable in the study of biological macromolecules and cellular structures.
Integration of High-Performance Computing
The integration of high-performance computing has been a game-changer in the field of electron microscopy. Advanced computational techniques enable researchers to process and analyze large datasets, extract meaningful information, and create detailed reconstructions of complex structures. This has led to significant advancements in fields such as structural biology, where the visualization of protein structures at atomic resolutions has been made possible.
- Image Processing: Enhancement and analysis of electron microscope images
- Data Analysis: Extraction of meaningful information from large datasets
- Simulation and Modeling: Prediction and visualization of complex systems
The use of AI-enhanced image processing has further expanded the capabilities of electron microscopy. Machine learning algorithms can automatically identify and classify features within images, enabling researchers to analyze large datasets with greater efficiency and accuracy. This has been particularly valuable in the study of complex biological systems, where the identification of specific structures and interactions can be challenging.
Ernst Ruska's Legacy and Influence
Ernst Ruska's invention of the electron microscope has had a lasting impact on the scientific community, spurring advancements in numerous fields and inspiring generations of researchers. His pioneering work has been recognized with numerous accolades, including the Nobel Prize in Physics in 1986, and his legacy continues to shape the future of scientific discovery.
Recognition and Awards
Throughout his career, Ernst Ruska received numerous awards and honors in recognition of his contributions to science. In addition to the Nobel Prize, he was awarded the Lasker Award in 1960 and the Paul Ehrlich and Ludwig Darmstaedter Prize in 1970. These accolades reflect the profound impact of his work on the scientific community and the broader world.
- Nobel Prize in Physics (1986)
- Lasker Award (1960)
- Paul Ehrlich and Ludwig Darmstaedter Prize (1970)
The Nobel Prize in Physics awarded to Ruska in 1986 was a testament to the transformative impact of his invention. The prize was shared with Gerd Binnig and Heinrich Rohrer for their work on scanning tunneling microscopy, highlighting the broader significance of advancements in imaging technology.
Influence on Future Generations
Ruska's work has inspired generations of scientists and engineers, encouraging them to push the boundaries of scientific discovery. His invention of the electron microscope has not only revolutionized imaging technology but also opened new avenues for exploration and innovation. Today, electron microscopy continues to evolve, incorporating cutting-edge technologies and driving advancements in fields ranging from biology to materials science.
- Education: Inspiring students and researchers to pursue careers in science and engineering
- Innovation: Encouraging the development of new technologies and techniques
- Collaboration: Fostering interdisciplinary research and cooperation
The influence of Ernst Ruska extends beyond his technical achievements. His commitment to scientific exploration and innovation has served as a model for future generations, encouraging them to pursue their own groundbreaking discoveries. The electron microscope, once a revolutionary invention, has become an indispensable tool in modern science, and its continued evolution is a testament to Ruska's enduring legacy.
The Future of Electron Microscopy
The field of electron microscopy continues to evolve, driven by advancements in technology and the ongoing pursuit of scientific discovery. Modern electron microscopes incorporate cutting-edge techniques such as cryo-electron microscopy, aberration-corrected lenses, and AI-enhanced image processing, enabling researchers to explore the microscopic world with unprecedented detail and precision.
Emerging Technologies
One of the most promising developments in electron microscopy is the advent of cryo-electron microscopy. This technique involves flash-freezing specimens to preserve their natural structures, allowing researchers to visualize biological macromolecules in their native states. This capability has been particularly valuable in the study of protein structures, enabling researchers to achieve sub-angstrom resolutions and gain insights into the fundamental mechanisms of biological processes.
- Cryo-Electron Microscopy: Visualization of biological macromolecules in their native states
- Aberration-Corrected Lenses: Enhancement of resolution and image quality
- AI-Enhanced Image Processing: Automatic identification and classification of features
The development of aberration-corrected lenses has also been a significant advancement in electron microscopy. These lenses correct for optical aberrations, enhancing the resolution and image quality of electron microscopes. This has enabled researchers to achieve unprecedented levels of detail, providing new insights into the structures and behaviors of materials at the atomic scale.
Applications in Nanotechnology
The field of nanotechnology has benefited greatly from the advancements in electron microscopy. The ability to visualize and manipulate materials at the nanoscale has led to the development of novel materials with unique properties, as well as a deeper understanding of the fundamental principles governing nanoscale phenomena. This has had a significant impact on industries ranging from electronics to medicine, driving innovations in areas such as nanomedicine, nanoelectronics, and nanomaterials.
- Nanomedicine: Development of targeted drug delivery systems and diagnostic tools
- Nanoelectronics: Creation of advanced electronic devices and components
- Nanomaterials: Exploration of materials with unique properties at the nanoscale
The future of electron microscopy holds great promise, with ongoing advancements in technology and technique driving new discoveries and innovations. As researchers continue to push the boundaries of what is possible, the legacy of Ernst Ruska and his groundbreaking invention will continue to inspire and shape the future of scientific exploration.
The Enduring Impact of Ernst Ruska's Electron Microscope
The electron microscope invented by Ernst Ruska has fundamentally transformed scientific research, enabling breakthroughs that were once unimaginable. From its humble beginnings in the 1930s to its modern iterations, this technology continues to push the boundaries of human knowledge, allowing scientists to explore the atomic and molecular worlds with remarkable precision.
Revolutionizing Scientific Research
The impact of the electron microscope on scientific research cannot be overstated. Before its invention, scientists were limited by the resolution of optical microscopes, which could only magnify objects up to about 2000x. Ruska's electron microscope shattered this barrier, achieving magnifications of 12,000x by 1933 and eventually reaching millions-fold magnification in modern systems. This leap in capability has unlocked new frontiers in fields such as biology, chemistry, and materials science.
- Biology: Enabled the visualization of viruses, cellular structures, and macromolecules
- Chemistry: Facilitated the study of molecular structures and chemical reactions at the atomic level
- Materials Science: Allowed for the analysis of crystalline structures, defects, and nanomaterials
One of the most significant contributions of the electron microscope has been in the field of virology. For the first time, scientists could visualize viral particles in intricate detail, leading to a deeper understanding of viral structures and their interactions with host cells. This knowledge has been crucial in the development of vaccines and antiviral therapies, ultimately saving countless lives.
Advancements in Medical Science
The electron microscope has played a pivotal role in advancing medical science. By enabling the visualization of cellular and sub-cellular structures, it has provided invaluable insights into the mechanisms of diseases and the development of targeted therapies. For example, the study of protein structures using electron microscopy has led to breakthroughs in understanding diseases such as Alzheimer's and Parkinson's.
- Disease Research: Visualization of pathogens and disease mechanisms
- Drug Development: Design of targeted therapies based on molecular structures
- Diagnostic Tools: Development of advanced imaging techniques for medical diagnostics
The advent of cryo-electron microscopy has further revolutionized medical research. This technique allows scientists to visualize biological macromolecules in their native states, providing unprecedented insights into their structures and functions. This capability has been instrumental in the development of new drugs and therapies, as well as in the understanding of complex biological processes.
Ernst Ruska's Influence on Modern Technology
Ernst Ruska's invention of the electron microscope has not only advanced scientific research but also had a profound impact on modern technology. The principles and techniques developed for electron microscopy have been applied to a wide range of technologies, from semiconductor manufacturing to nanotechnology. This section explores the various ways in which Ruska's work has shaped the technological landscape.
Semiconductor Industry
The semiconductor industry has greatly benefited from the advancements in electron microscopy. The ability to visualize and manipulate materials at the atomic scale has been crucial in the development of integrated circuits and other electronic components. Electron microscopy has enabled engineers to analyze the structure and properties of semiconductor materials, leading to the creation of more efficient and powerful electronic devices.
- Integrated Circuits: Analysis and optimization of semiconductor structures
- Material Characterization: Study of material properties and defects
- Quality Control: Inspection and testing of electronic components
The use of scanning electron microscopy (SEM) and transmission electron microscopy (TEM) has become standard practice in the semiconductor industry. These techniques allow for the detailed analysis of semiconductor materials, enabling engineers to identify and correct defects, optimize performance, and develop new technologies.
Nanotechnology
The field of nanotechnology has been particularly transformed by the advancements in electron microscopy. The ability to visualize and manipulate materials at the nanoscale has led to the development of novel materials with unique properties, as well as the creation of advanced nanodevices. Electron microscopy has been instrumental in the study of nanomaterials, enabling researchers to explore their structures, properties, and behaviors.
- Nanomaterials: Exploration of materials with unique properties at the nanoscale
- Nanodevices: Development of advanced devices and components
- Nanoelectronics: Creation of electronic devices at the nanoscale
The development of aberration-corrected lenses has further enhanced the capabilities of electron microscopy in nanotechnology. These lenses correct for optical aberrations, enabling researchers to achieve unprecedented levels of detail and precision. This has led to significant advancements in the study of nanomaterials and the development of nanodevices, driving innovations in fields such as nanoelectronics and nanomedicine.
Preserving Ernst Ruska's Legacy
The legacy of Ernst Ruska and his groundbreaking invention continues to inspire and shape the future of scientific discovery. His work has been preserved and celebrated in various ways, ensuring that his contributions to science and technology are remembered and appreciated by future generations.
Museums and Exhibitions
The original electron microscope developed by Ruska is preserved at the Deutsches Museum in Munich, serving as a testament to his ingenuity and innovation. This historic artifact is a reminder of the transformative impact of Ruska's work and the enduring legacy of his invention. Museums and exhibitions around the world continue to showcase the evolution of electron microscopy, highlighting its significance in the history of science and technology.
- Deutsches Museum: Preservation of the original electron microscope
- Science Museums: Exhibitions on the history and evolution of electron microscopy
- Educational Programs: Initiatives to inspire future generations of scientists and engineers
Educational programs and initiatives have been developed to inspire future generations of scientists and engineers. These programs aim to foster a deeper understanding of the principles and applications of electron microscopy, encouraging students to pursue careers in science and technology. By preserving and promoting Ruska's legacy, these initiatives ensure that his contributions continue to inspire and shape the future of scientific discovery.
Educational Resources and Outreach
The importance of educational resources and outreach in preserving Ernst Ruska's legacy cannot be overstated. Educational videos, online courses, and interactive exhibits have been developed to provide students and researchers with a comprehensive understanding of electron microscopy and its applications. These resources aim to inspire and engage the next generation of scientists, ensuring that Ruska's work continues to have a lasting impact.
- Online Courses: Comprehensive courses on electron microscopy and its applications
- Interactive Exhibits: Hands-on experiences to explore the principles of electron microscopy
- Educational Videos: Engaging content to inspire and educate students and researchers
One notable example of educational outreach is the series of videos produced in 2023, which highlight the evolution of electron microscopy from Ruska's early prototypes to advanced techniques such as scanning electron microscopy (SEM) and transmission electron microscopy (TEM). These videos provide a compelling overview of the history and significance of electron microscopy, inspiring students and researchers to explore the microscopic world.
Conclusion: The Lasting Legacy of Ernst Ruska
Ernst Ruska's invention of the electron microscope has had a profound and lasting impact on the scientific community. His pioneering work has enabled researchers to explore the microscopic world with unprecedented detail and precision, driving advancements in fields ranging from biology to materials science. The electron microscope has become an indispensable tool in modern science, and its continued evolution is a testament to Ruska's enduring legacy.
Key Takeaways
The key takeaways from Ernst Ruska's contributions to science and technology are as follows:
- Revolutionary Invention: The electron microscope shattered the resolution barriers of optical microscopes, achieving magnifications of millions-fold.
- Transformative Impact: Enabled breakthroughs in biology, chemistry, materials science, and medical research.
- Technological Advancements: Drove innovations in semiconductor manufacturing, nanotechnology, and advanced imaging techniques.
- Inspiration for Future Generations: Ruska's work continues to inspire and shape the future of scientific discovery.
The electron microscope has not only revolutionized scientific research but also had a profound impact on modern technology. From the development of advanced electronic devices to the exploration of nanomaterials, Ruska's invention has driven innovations that have transformed industries and improved lives. His legacy serves as a reminder of the power of scientific curiosity and the potential for groundbreaking discoveries to shape the future.
The Future of Electron Microscopy
The future of electron microscopy holds great promise, with ongoing advancements in technology and technique driving new discoveries and innovations. Modern electron microscopes incorporate cutting-edge technologies such as high-performance computing, AI-enhanced image processing, and advanced imaging techniques, enabling researchers to explore the microscopic world with unprecedented detail and precision.
- Cryo-Electron Microscopy: Visualization of biological macromolecules in their native states.
- Aberration-Corrected Lenses: Enhancement of resolution and image quality.
- AI-Enhanced Image Processing: Automatic identification and classification of features.
As researchers continue to push the boundaries of what is possible, the legacy of Ernst Ruska and his groundbreaking invention will continue to inspire and shape the future of scientific exploration. The electron microscope, once a revolutionary invention, has become an indispensable tool in modern science, and its continued evolution is a testament to Ruska's enduring impact on the world of science and technology.
In conclusion, Ernst Ruska's contributions to science and technology have left an indelible mark on the world. His invention of the electron microscope has revolutionized scientific research, driven technological advancements, and inspired generations of scientists and engineers. As we look to the future, the continued evolution of electron microscopy serves as a reminder of the power of innovation and the potential for groundbreaking discoveries to transform our understanding of the world.