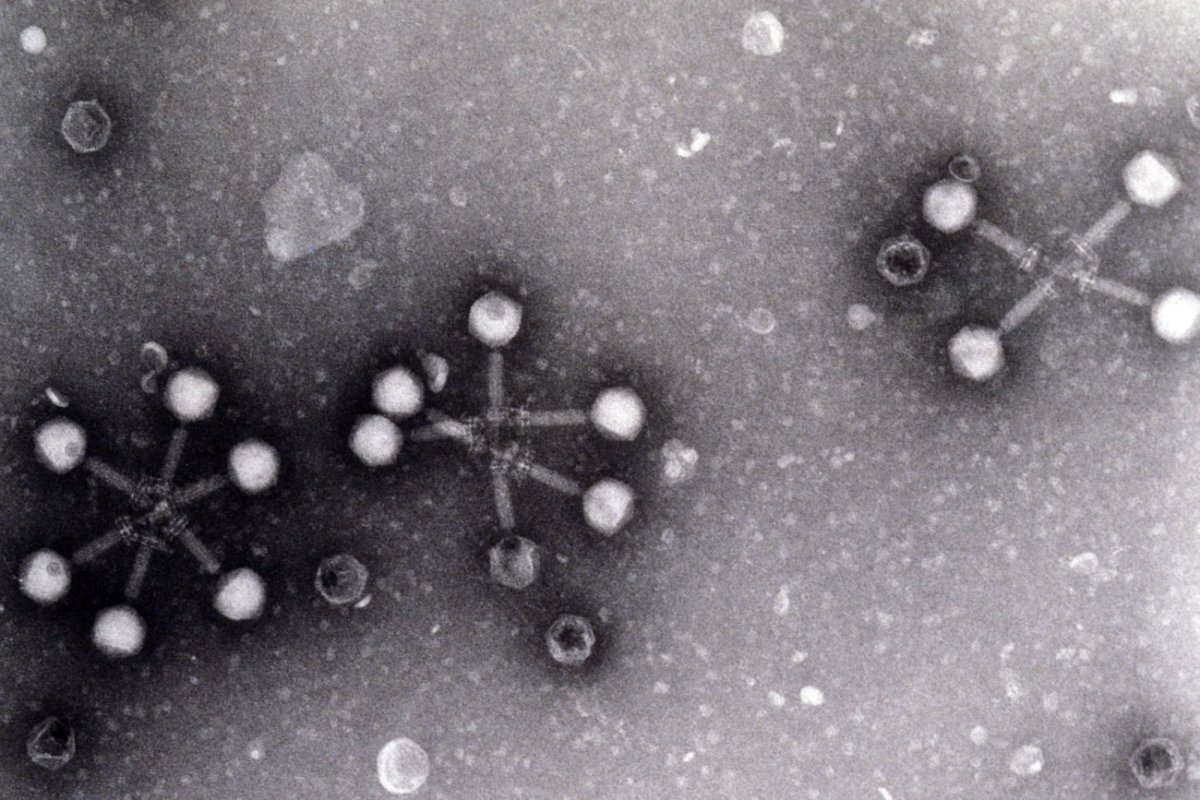
Felix d Herelle: Pioneer of Bacteriophages
The story of Félix d'Hérelle is one of unconventional genius. Born in Montreal in 1873, this French-Canadian microbiologist revolutionized science with a discovery that would shape modern medicine and molecular biology. Félix d'Hérelle is celebrated as the co-discoverer of bacteriophages, the viruses that infect bacteria. Despite having only a high school education, his pioneering work in phage therapy and biological pest control cemented his legacy.
His journey from self-taught scientist to world-renowned researcher is a testament to sharp observation and intellectual daring. D'Hérelle's work laid the foundation for entire fields of study, from virology to genetic engineering.
The Unlikely Path of a Microbiological Genius
Félix d'Hérelle's early life did not predict a future as a scientific luminary. His formal education ended with high school. Yet, an intense curiosity about the natural world drove him to teach himself microbiology. This self-directed learning became the cornerstone of a remarkable career that defied the academic norms of his era.
He began his practical work far from Europe's prestigious institutes. D'Hérelle served as a bacteriologist at the General Hospital in Guatemala City. There, he organized public health defenses against deadly diseases like malaria and yellow fever.
From Sisal to Locusts: A Pivotal Assignment
D'Hérelle's path to discovery took a decisive turn in Mexico. Initially, he was tasked with studying the alcoholic fermentation of sisal residue. This industrial project unexpectedly led him into the world of insect pathology.
While investigating diseases affecting locusts, he made a critical observation. On agar cultures of bacteria infecting the insects, he noticed clear spots where the bacterial lawn had been wiped out. This simple observation sparked the idea of using pathogens to control pests.
Joining the Pasteur Institute and Early Recognition
In 1911, d'Hérelle's growing expertise earned him a position at the famed Pasteur Institute in Paris. He started as an unpaid assistant, yet his talent quickly shone. He gained international attention for his successful campaigns against Mexican locust plagues.
He utilized a bacterium called Coccobacillus to devastate locust populations. This work established him as an innovative thinker in applied microbiology. It also foreshadowed his future title as the "father of biological pest control."
His methods represented a groundbreaking approach to agriculture. They preceded modern biocontrol agents like Bacillus thuringiensis (Bt) by decades. The stage was now set for his most profound contribution to science.
The Groundbreaking Discovery of Bacteriophages
The year 1917 marked a watershed moment in microbiology. On September 10, Félix d'Hérelle published a landmark note in the Comptes rendus de l'Academie des sciences. He described a mysterious "obligate intracellular parasite" of bacteria. This discovery would define his career and alter the course of biological science.
The discovery occurred during World War I. D'Hérelle was investigating a severe dysentery outbreak afflicting a French cavalry squadron. He filtered bacterial cultures from sick soldiers and observed something extraordinary.
The filtrate, even when diluted, could rapidly and completely destroy cultures of dysentery bacteria. D'Hérelle termed the invisible agent a "bacteria-eater," or bacteriophage.
Methodological Brilliance in Virology
D'Hérelle's genius extended beyond the initial observation. He developed a simple yet powerful technique to quantify these invisible entities. He serially diluted suspensions containing the phage and spread them on bacterial lawns.
Instead of uniformly killing the bacteria, the highest dilutions created discrete, clear spots called plaques. D'Hérelle reasoned correctly that each plaque originated from a single viral particle.
- He counted the plaques on the most diluted sample.
- He multiplied that count by the dilution factor.
- This calculation gave him the number of bacteriophage viruses in his original suspension.
This method established the foundational plaque assay, a technique still central to virology today. Between 1918 and 1921, he identified different phages targeting various bacterial species, including the deadly Vibrio cholerae.
A Note on Precedence: Twort vs. d'Hérelle
History notes that British microbiologist F.W. Twort observed a similar phenomenon in 1915. However, Twort was hesitant to pursue or promote his finding. D'Hérelle's systematic investigation, relentless promotion, and coining of the term "bacteriophage" made his work the definitive cornerstone of the field.
His discovery provided the first clear evidence of viruses that could kill bacteria. This opened a new frontier in the battle against infectious disease.
The Dawn of Phage Therapy
Félix d'Hérelle was not content with mere discovery. He immediately envisioned a therapeutic application. He pioneered phage therapy, the use of bacteriophages to treat bacterial infections. His first successful experiment was dramatic.
In early 1919, he isolated phages from chicken feces. He used them to treat a virulent chicken typhus plague, saving the birds. This success in animals gave him the confidence to attempt human treatment.
The first human trial occurred in August 1919. D'Hérelle successfully treated a patient suffering from severe bacterial dysentery using his phage preparations. This milestone proved the concept that viruses could be used as healers.
He consolidated his findings in his 1921 book, Le bactériophage, son rôle dans l'immunité ("The Bacteriophage, Its Role in Immunity"). This work firmly established him as the father of phage therapy. The potential for a natural, self-replicating antibiotic alternative was now a reality.
Global Impact and Controversies of Phage Therapy
The success of d'Hérelle's initial human trial catapulted phage therapy into the global spotlight. Doctors worldwide began experimenting with bacteriophages to combat a range of bacterial infections. This period marked the first major application of virology in clinical medicine.
D'Hérelle collaborated with the pharmaceutical company L'Oréal to produce and distribute phage preparations. Their products targeted dysentery, cholera, and plague, saving countless lives. This commercial partnership demonstrated the immense therapeutic potential he had unlocked.
However, the rapid adoption of phage therapy was not without significant challenges. The scientific understanding of bacteriophage biology was still in its infancy. These inconsistencies led to skeptical reactions from parts of the medical establishment.
The Soviet Union Embraces Phage Research
While Western medicine grew cautious, the Soviet Union enthusiastically adopted d'Hérelle's work. In 1923, he was invited to Tbilisi, Georgia, by microbiologist George Eliava. This collaboration led to the founding of the Eliava Institute of Bacteriophage.
The Institute became a global epicenter for phage therapy research and application. It treated Red Army soldiers during World War II, using phages to prevent gangrene and other battlefield infections. To this day, the institute remains a leading facility for phage therapy.
The partnership between d'Hérelle and Eliava was scientifically fruitful but ended tragically. George Eliava was executed in 1937 during Stalin's Great Purge, a severe blow to their shared vision.
Challenges in the West
In Europe and North America, phage therapy faced a more skeptical reception. Early clinical studies often produced inconsistent results due to several critical factors that were not yet understood.
- Poor Phage Purification: Early preparations often contained bacterial debris, causing adverse reactions in patients.
- Phage Specificity: Doctors did not always match the specific phage to the specific bacterial strain causing the infection.
- Bacterial Resistance: The ability of bacteria to develop resistance to phages was not fully appreciated.
The discovery and mass production of chemical antibiotics like penicillin in the 1940s further sidelined phage therapy in the West. Antibiotics were easier to standardize and had a broader spectrum of activity. For decades, phage therapy became a largely Eastern European practice.
Expanding the Scope: Public Health and Biological Control
Félix d'Hérelle's vision for bacteriophages extended far beyond individual patient treatment. He was a pioneering thinker in the field of public health. He saw phages as a tool for preventing disease on a massive scale.
He conducted large-scale experiments to prove that bacteriophages could be used to sanitize water supplies. By introducing specific phages into wells and reservoirs, he aimed to eliminate waterborne pathogens like cholera. This proactive approach was revolutionary for its time.
Combating Cholera Epidemics
D'Hérelle applied his public health philosophy to combat real-world epidemics. He traveled to India in the late 1920s to fight cholera, a disease that ravaged the population. His work there demonstrated the potential for community-wide prophylaxis.
He administered phage preparations to thousands of individuals in high-risk communities. His efforts showed a significant reduction in cholera incidence among those treated. This large-scale application provided compelling evidence for the power of phage-based prevention.
Despite these successes, logistical challenges and the rise of alternative public health measures limited widespread adoption. Yet, his work remains a landmark in the history of epidemiological intervention.
Return to Biological Pest Control
D'Hérelle never abandoned his early interest in using microbes against insect pests. His discovery of bacteriophages reinforced his belief in biological solutions. He continued to advocate for the use of pathogens to control agricultural threats.
His early success with Coccobacillus against locusts paved the way for modern biocontrol. This approach is now a cornerstone of integrated pest management. It reduces the reliance on chemical pesticides, benefiting the environment.
D'Hérelle is rightly credited as a founding father of this field. His ideas directly anticipated the development and use of Bacillus thuringiensis (Bt), a bacterium used worldwide as a natural insecticide.
Scientific Recognition and Academic Pursuits
Despite his lack of formal academic credentials, Félix d'Hérelle achieved remarkable recognition. His groundbreaking discoveries could not be ignored by the scientific community. He received numerous honors and prestigious appointments.
In 1924, the University of Leiden in the Netherlands appointed him a professor. This was a significant achievement for a self-taught scientist. He also received an honorary doctorate from the University of Leiden, validating his contributions to science.
His work earned him a nomination for the Nobel Prize in Physiology or Medicine. Although he never won, the nomination itself placed him among the most elite researchers of his generation. His legacy was secured by the profound impact of his discoveries.
The Nature of Viruses and Theoretical Contributions
D'Hérelle was not just an experimentalist; he was also a theorist who pondered the fundamental nature of life. He engaged in spirited debates about whether bacteriophages were living organisms or complex enzymes. He passionately argued that they were living viruses.
His theories on immunity were also advanced. He proposed that bacteriophages played a crucial role in natural immunity. He suggested that the body's recovery from bacterial infections was often mediated by the natural activity of these viruses.
- Theory of Natural Immunity: D'Hérelle believed phages in the environment provided a first line of defense.
- Debate on Viral Life: His arguments helped shape the early field of virology.
- Host-Parasite Relationship: He provided a clear model for understanding obligate parasitism.
These theoretical battles were vital for the development of microbiology. They forced the scientific community to confront and define the boundaries of life at the microscopic level.
Later Career and Move to Yale
In 1928, d'Hérelle accepted a position at Yale University in the United States. This move signaled his high standing in American academic circles. At Yale, he continued his research and mentored a new generation of scientists.
His later work focused on refining phage therapy techniques and understanding phage genetics. He continued to publish prolifically, sharing his findings with the world. However, his unwavering and sometimes stubborn adherence to his own theories occasionally led to friction with colleagues.
Despite these interpersonal challenges, his productivity remained high. His time at Yale further cemented the importance of bacteriophage research in American institutions.
Later Years and Scientific Legacy
Félix d'Hérelle remained an active and prolific researcher well into his later years. After his tenure at Yale University, he returned to France, continuing his work with undiminished passion. He maintained a laboratory in Paris, where he pursued his investigations into viruses and their applications.
Despite facing occasional isolation from the mainstream scientific community due to his strong-willed nature, his dedication never wavered. He continued to write and publish, defending his theories and promoting the potential of bacteriophages. His later writings reflected a lifetime of observation and a deep belief in the power of biological solutions.
D'Hérelle passed away in Paris on February 22, 1949, from pancreatic cancer. His death marked the end of a remarkable life dedicated to scientific discovery. He left behind a legacy that would only grow in significance with time.
The Modern Revival of Phage Therapy
For decades after the antibiotic revolution, phage therapy was largely forgotten in the West. However, the late 20th and early 21st centuries have witnessed a dramatic resurgence of interest. The driving force behind this revival is the global crisis of antibiotic resistance.
As multidrug-resistant bacteria like MRSA and CRE have become major public health threats, scientists have returned to d'Hérelle's work. Phage therapy offers a promising alternative or complement to traditional antibiotics. Modern clinical trials are now validating many of his early claims with rigorous scientific methods.
- Personalized Medicine: Phages can be tailored to target specific bacterial strains infecting a patient.
- Fewer Side Effects: Phages are highly specific, reducing damage to the body's beneficial microbiome.
- Self-Replicating Treatment: Phages multiply at the site of infection until the host bacteria are eliminated.
Research institutions worldwide, including in the United States and Western Europe, are now investing heavily in phage research. This represents a full-circle moment for d'Hérelle's pioneering vision.
Foundation of Molecular Biology
Perhaps d'Hérelle's most profound, though indirect, legacy is his contribution to the birth of molecular biology. In the 1940s and 1950s, bacteriophages became the model organism of choice for pioneering geneticists.
The "Phage Group," led by scientists like Max Delbrück and Salvador Luria, used phages to unravel the fundamental principles of life. Their experiments with phage replication and genetics answered critical questions about how genes function and how DNA operates as the genetic material.
Key discoveries like the mechanism of DNA replication, gene regulation, and the structure of viruses were made using bacteriophages. The 1969 Nobel Prize in Physiology or Medicine was awarded to Delbrück, Luria, and Herschel for their work on phage genetics.
This means that the tools and knowledge that underpin modern biotechnology and genetic engineering can trace their origins back to d'Hérelle's initial isolation and characterization of these viruses. He provided the raw material for a scientific revolution.
Honors, Recognition, and Lasting Tributes
Although Félix d'Hérelle did not receive a Nobel Prize, his work earned him numerous other prestigious accolades during his lifetime. These honors acknowledged the transformative nature of his discoveries.
He was awarded the Leeuwenhoek Medal by the Royal Netherlands Academy of Arts and Sciences in 1925. This medal, awarded only once every decade, is considered the highest honor in microbiology. It recognized him as the most significant microbiologist of his era.
He was also made an honorary member of numerous scientific societies across Europe and North America. These memberships were a testament to the international respect he commanded, despite his unconventional background.
The Eliava Institute: A Living Legacy
The most enduring tribute to d'Hérelle's work is the Eliava Institute of Bacteriophage, Microbiology, and Virology in Tbilisi, Georgia. Founded with his close collaborator George Eliava, the institute has remained a global leader in phage therapy for over a century.
While the Western world abandoned phage therapy for antibiotics, the Eliava Institute continued to treat patients and refine its techniques. Today, it attracts patients from around the globe who have infections untreatable by conventional antibiotics.
The institute stands as a physical monument to d'Hérelle's vision. It continues his mission of healing through the intelligent application of natural biological agents.
Conclusion: The Enduring Impact of Félix d'Hérelle
Félix d'Hérelle's story is a powerful reminder that revolutionary ideas can come from outside established systems. His lack of formal academic training did not hinder his ability to see what others missed. His greatest strength was his power of observation and his willingness to follow the evidence wherever it led.
He was a true pioneer who entered uncharted scientific territory. His discovery of bacteriophages opened up multiple new fields of study. From medicine to agriculture to genetics, his influence is deeply woven into the fabric of modern science.
Key Takeaways from a Revolutionary Career
The life and work of Félix d'Hérelle offer several critical lessons for science and innovation.
- Curiosity Drives Discovery: A simple observation of clear spots on a细菌 lawn led to a world-changing breakthrough.
- Application is Key: D'Hérelle immediately sought to apply his discovery to solve real-world problems like disease and famine.
- Persistence Overcomes Skepticism: He championed his ideas relentlessly, even when faced with doubt from the establishment.
- Interdisciplinary Vision: He effortlessly connected microbiology with medicine, public health, and agriculture.
His career demonstrates that the most significant scientific contributions often defy traditional boundaries and expectations.
A Legacy for the Future
Today, as we confront the looming threat of a post-antibiotic era, d'Hérelle's work is more relevant than ever. Phage therapy is being re-evaluated as a crucial weapon in the fight against superbugs. Research into using phages in food safety and agriculture is also expanding.
Furthermore, bacteriophages continue to be indispensable tools in laboratories worldwide. They are used in genetic engineering, synthetic biology, and basic research. The field of molecular biology, which they helped create, continues to transform our world.
Félix d'Hérelle's legacy is not confined to the history books. It is a living, evolving force in science and medicine. From a self-taught microbiologist in Guatemala to a father of modern virology, his journey proves that a single curious mind can indeed change the world. His story inspires us to look closely, think boldly, and harness the power of nature to heal and protect.


Alexander Fleming: The Pioneer of the Antibiotic Revolution
In the annals of medical history, few discoveries have had as profound an impact as Alexander Fleming's discovery of penicillin in 1928. This Scottish physician and microbiologist inadvertently sparked the antibiotic revolution, forever changing the landscape of modern medicine. His groundbreaking work not only introduced the world's first broadly effective antibiotic but also laid the foundation for the development of countless life-saving drugs. This article delves into the life, discoveries, and enduring legacy of Alexander Fleming, the man who transformed our ability to combat infectious diseases.
The Early Life and Career of Alexander Fleming
Born on August 6, 1881, in Lochfield, Scotland, Alexander Fleming grew up in a rural setting that would later influence his scientific curiosity. After completing his education at St. Mary's Hospital Medical School in London, Fleming embarked on a career in microbiology, driven by a desire to understand and combat bacterial infections.
Education and Early Influences
Fleming's academic journey began at the University of London, where he studied medicine. His early exposure to the works of Louis Pasteur and Robert Koch ignited his passion for bacteriology. These pioneers in microbiology inspired Fleming to explore the intricate world of bacteria and their role in human health.
Military Service and Post-War Research
During World War I, Fleming served as a captain in the Royal Army Medical Corps. His experiences on the battlefield, where he witnessed the devastating effects of bacterial infections on wounded soldiers, deepened his resolve to find effective treatments. Post-war, he returned to St. Mary's Hospital, where he continued his research on antibacterial substances.
The Discovery of Lysozyme: A Prelude to Penicillin
Before his monumental discovery of penicillin, Fleming made another significant contribution to microbiology with the identification of lysozyme in 1922. This enzyme, found in tears, saliva, and mucus, exhibited weak antibacterial properties against certain non-pathogenic bacteria.
The Significance of Lysozyme
Although lysozyme's antibacterial effects were limited, its discovery was crucial for several reasons:
- It demonstrated the existence of natural antibacterial substances within the human body.
- It provided insights into the body's innate defense mechanisms against bacterial infections.
- It set the stage for Fleming's later, more impactful discovery of penicillin.
Experimental Observations
Fleming's meticulous observations during his experiments with lysozyme highlighted his keen eye for detail. He noted that the enzyme could dissolve certain bacteria, albeit not the more harmful pathogens. This work underscored the potential for discovering more potent antibacterial agents, a pursuit that would soon lead him to penicillin.
The Serendipitous Discovery of Penicillin
The story of penicillin's discovery is one of scientific serendipity. In 1928, while studying Staphylococcus aureus at St. Mary's Hospital, Fleming noticed something unusual in one of his Petri dishes. A mold, later identified as Penicillium notatum, had contaminated the dish and inhibited the growth of bacteria around it.
The Contaminated Petri Dish
Fleming's laboratory was known for its somewhat disorganized state, a characteristic that ironically played a role in his discovery. An uncovered Petri dish near an open window became contaminated with mold spores. Instead of discarding the dish, Fleming observed that the bacteria near the mold were being destroyed. This observation led him to isolate the antibacterial substance, which he named penicillin on March 7, 1929.
Initial Reactions and Challenges
Despite the promising nature of his discovery, Fleming's initial publication in 1929 garnered little attention. The scientific community was skeptical, and the instability of penicillin posed significant challenges for its practical application. It would take over a decade for the full potential of penicillin to be realized, thanks to the efforts of Howard Florey and Ernst Chain.
Collaborators in the Antibiotic Revolution
While Alexander Fleming is credited with the discovery of penicillin, the development of the antibiotic into a viable medical treatment involved the collaborative efforts of several key figures. Among them, Howard Florey and Ernst Chain played pivotal roles in purifying penicillin and demonstrating its efficacy.
Howard Florey: The Driving Force Behind Purification
Howard Florey, an Australian pharmacologist, led the team at the University of Oxford that successfully purified penicillin. His relentless efforts in the late 1930s and early 1940s transformed Fleming's "mold juice" into a stable, usable antibiotic. Florey's work was instrumental in scaling up production and conducting the first clinical trials.
Ernst Chain: The Biochemist's Contribution
Ernst Chain, a German-born biochemist, collaborated closely with Florey. His expertise in biochemistry was crucial in isolating and concentrating penicillin. Chain's contributions ensured that the antibiotic could be produced in quantities sufficient for medical use, paving the way for its widespread adoption.
The Nobel Prize and Recognition
In 1945, Alexander Fleming, Howard Florey, and Ernst Chain were jointly awarded the Nobel Prize in Physiology or Medicine for their groundbreaking work on penicillin. This recognition underscored the collaborative nature of scientific discovery and the profound impact of their contributions to medicine.
The Impact of Penicillin on World War II
The advent of World War II provided a critical impetus for the mass production of penicillin. The urgent need for effective treatments for infected wounds and diseases among soldiers accelerated the development and distribution of the antibiotic.
Early Clinical Trials and Successes
The first human trial of penicillin took place in 1941, with a policeman named Albert Alexander. Although the initial results were promising, supply shortages limited the treatment's effectiveness. However, subsequent trials demonstrated penicillin's remarkable ability to combat a range of bacterial infections, including streptococcal, staphylococcal, and gonococcal infections.
Mass Production and Military Use
By 1942, the United States had established pilot plants for the mass production of penicillin. Companies like Merck played a crucial role in scaling up production, ensuring that the antibiotic was available in sufficient quantities for military use. Penicillin became a vital tool in treating wounded soldiers, significantly reducing mortality rates from infections.
Post-War Impact and Public Health
The success of penicillin during the war set the stage for its post-war adoption in public health. The antibiotic's effectiveness against diseases such as syphilis, pneumonia, and meningitis revolutionized medical practice. Penicillin's impact extended beyond the battlefield, transforming the treatment of bacterial infections worldwide.
Alexander Fleming's Legacy and the Antibiotic Era
The discovery of penicillin marked the beginning of the antibiotic era, a period characterized by the development and use of antibiotics to combat bacterial infections. Fleming's work laid the foundation for modern antibiotic therapy, saving countless lives and shaping the course of medical history.
The Foundation of Modern Antibiotics
Penicillin's success inspired the discovery and development of numerous other antibiotics. Drugs like streptomycin, tetracycline, and erythromycin followed, each contributing to the arsenal of treatments available to combat bacterial infections. The antibiotic era has been marked by continuous innovation, driven by the principles established by Fleming's discovery.
Challenges and the Rise of Antibiotic Resistance
Despite the transformative impact of antibiotics, their use has not been without challenges. Alexander Fleming himself warned of the potential for antibiotic resistance as early as 1942. His concerns have proven prescient, as the rise of multidrug-resistant bacteria poses a significant threat to global health. The ongoing battle against antibiotic resistance underscores the need for continued research and innovation in the field of microbiology.
Honoring Fleming's Contributions
Today, Alexander Fleming is remembered as a pioneer in the field of microbiology. His discovery of penicillin has earned him a place among the most influential figures in medical history. Institutions and organizations worldwide continue to honor his legacy, recognizing the profound impact of his work on human health and well-being.
In the next part of this article, we will delve deeper into the scientific details of penicillin's discovery, its mechanism of action, and the ongoing efforts to combat antibiotic resistance. Stay tuned for an exploration of the intricate world of antibiotics and the enduring legacy of Alexander Fleming.
The Science Behind Penicillin: Mechanism and Development
The discovery of penicillin by Alexander Fleming was a pivotal moment in medical history, but understanding its mechanism of action and the subsequent development process is equally fascinating. This section explores the scientific intricacies of penicillin, from its antibacterial properties to the challenges faced in its mass production.
How Penicillin Works: A Bactericidal Agent
Penicillin operates as a bactericidal agent, meaning it kills bacteria rather than merely inhibiting their growth. Its primary mechanism involves interfering with the synthesis of the bacterial cell wall. Specifically, penicillin targets the enzyme transpeptidase, which is crucial for cross-linking the peptide chains in the bacterial cell wall.
When penicillin binds to transpeptidase, it prevents the formation of a strong and rigid cell wall. This weakening leads to the bacteria becoming susceptible to osmotic pressure, ultimately causing the cell to lyse (burst) and die. This mode of action is particularly effective against Gram-positive bacteria, which have a thick cell wall composed primarily of peptidoglycan.
The Challenges of Early Penicillin Production
Despite its promising antibacterial properties, the early production of penicillin faced numerous challenges. Fleming's initial "mold juice" was highly unstable and difficult to purify. The key obstacles included:
- Instability: Penicillin degraded rapidly, making it challenging to store and use effectively.
- Low Yield: The mold Penicillium notatum produced only small amounts of penicillin, insufficient for medical use.
- Purification Difficulties: Isolating pure penicillin from the mold broth was a complex and time-consuming process.
These challenges necessitated innovative solutions, which were ultimately provided by Howard Florey and Ernst Chain at the University of Oxford.
From Laboratory Discovery to Mass Production
The journey of penicillin from a laboratory curiosity to a widely available antibiotic is a testament to the power of scientific collaboration and innovation. This section delves into the critical steps that transformed penicillin into a medical marvel.
The Oxford Team's Breakthrough
In the late 1930s, Howard Florey and Ernst Chain took up the challenge of purifying and stabilizing penicillin. Their work at the University of Oxford marked a turning point in the antibiotic's development. By 1940, they had successfully produced a purified form of penicillin that was stable enough for clinical trials.
The Oxford team's breakthrough involved several key innovations:
- Improved Cultivation Techniques: They developed methods to grow Penicillium notatum in large quantities, increasing the yield of penicillin.
- Advanced Purification Processes: Using techniques such as chromatography, they isolated pure penicillin from the mold broth.
- Stabilization Methods: They found ways to stabilize penicillin, making it suitable for storage and medical use.
The First Clinical Trials and Human Use
The first human trial of penicillin took place on February 12, 1941, with a patient named Albert Alexander. Alexander, a policeman, was suffering from severe infections caused by Staphylococcus aureus. The trial demonstrated penicillin's remarkable efficacy, as Alexander showed significant improvement shortly after receiving the treatment.
However, the initial success was tempered by the limited supply of penicillin. Despite the Oxford team's efforts, they could not produce enough penicillin to sustain Alexander's treatment, and he ultimately relapsed. This experience underscored the urgent need for large-scale production of the antibiotic.
Mass Production During World War II
The onset of World War II provided the necessary impetus for the mass production of penicillin. The United States, recognizing the antibiotic's potential to save lives on the battlefield, invested heavily in scaling up production. Key developments during this period included:
- Industrial Collaboration: Pharmaceutical companies such as Merck and Pfizer established pilot plants for penicillin production.
- Innovative Fermentation Techniques: Scientists developed deep-tank fermentation methods, significantly increasing the yield of penicillin.
- Government Support: The U.S. government funded research and production efforts, ensuring that penicillin was available in sufficient quantities for military use.
By 1944, penicillin was being produced in large quantities, with hundreds of liters available weekly. This mass production effort was instrumental in treating wounded soldiers and reducing mortality rates from bacterial infections.
The Impact of Penicillin on Modern Medicine
The introduction of penicillin revolutionized the field of medicine, transforming the treatment of bacterial infections and saving countless lives. This section explores the profound impact of penicillin on modern medical practice and public health.
Revolutionizing the Treatment of Bacterial Infections
Before the advent of penicillin, bacterial infections were a leading cause of death worldwide. Diseases such as pneumonia, syphilis, and meningitis often proved fatal due to the lack of effective treatments. Penicillin changed this landscape dramatically, providing a powerful tool to combat a wide range of bacterial infections.
Some of the key infections treated by penicillin include:
- Streptococcal Infections: Including strep throat and scarlet fever.
- Staphylococcal Infections: Such as skin infections and abscesses.
- Gonococcal Infections: Including gonorrhea, a common sexually transmitted infection.
- Syphilis: A previously devastating disease that could now be effectively treated.
Penicillin's Role in Surgery and Wound Care
The impact of penicillin extended beyond the treatment of systemic infections. The antibiotic played a crucial role in surgical practice and wound care, significantly reducing the risk of post-operative infections. Before penicillin, surgical procedures carried a high risk of complications due to bacterial contamination. With the advent of penicillin, surgeons could perform operations with greater confidence, knowing that infections could be effectively managed.
During World War II, penicillin was extensively used to treat wounded soldiers, preventing infections that would have otherwise been fatal. This application not only saved lives but also demonstrated the antibiotic's versatility and efficacy in a range of medical settings.
The Foundation for Antibiotic Research
The success of penicillin inspired a wave of research into other antibiotics. Scientists around the world began exploring the potential of natural and synthetic compounds to combat bacterial infections. This research led to the discovery of numerous antibiotics, each with unique properties and applications.
Some of the notable antibiotics developed in the wake of penicillin include:
- Streptomycin: Effective against tuberculosis and other Gram-negative bacteria.
- Tetracycline: A broad-spectrum antibiotic used to treat a variety of infections.
- Erythromycin: An alternative for patients allergic to penicillin.
The discovery of these antibiotics expanded the arsenal of treatments available to medical professionals, further enhancing their ability to combat bacterial infections.
Alexander Fleming's Warnings and the Rise of Antibiotic Resistance
Despite the transformative impact of penicillin, Alexander Fleming was acutely aware of the potential for antibiotic resistance. As early as 1942, he warned that the overuse and misuse of antibiotics could lead to the development of resistant bacterial strains. This section explores Fleming's prescient warnings and the ongoing challenge of antibiotic resistance.
Fleming's Early Observations on Resistance
In his Nobel Prize acceptance speech, Fleming cautioned about the dangers of antibiotic resistance:
"It is not difficult to make microbes resistant to penicillin in the laboratory by exposing them to concentrations not sufficient to kill them, and the same thing has occasionally happened in the body."
Fleming's observations were based on his experiments, where he noted that bacteria exposed to sub-lethal doses of penicillin could develop resistance. This phenomenon, known as antibiotic resistance, occurs when bacteria evolve mechanisms to survive the effects of antibiotics.
The Emergence of Resistant Bacterial Strains
The first cases of penicillin-resistant bacteria were documented in the early 1940s, shortly after the antibiotic's introduction. One of the most notable examples is Staphylococcus aureus, a common pathogen that quickly developed resistance to penicillin. Today, methicillin-resistant Staphylococcus aureus (MRSA) is a significant public health concern, causing infections that are difficult to treat with standard antibiotics.
The rise of antibiotic resistance is driven by several factors, including:
- Overuse of Antibiotics: The excessive prescription of antibiotics for viral infections, which they cannot treat, contributes to resistance.
- Incomplete Treatment Courses: Patients who do not complete their prescribed antibiotic courses allow bacteria to survive and develop resistance.
- Agricultural Use: The use of antibiotics in livestock farming accelerates the development of resistant strains.
The Global Crisis of Antibiotic Resistance
Today, antibiotic resistance is recognized as a global health crisis. The World Health Organization (WHO) has warned that without urgent action, we could enter a post-antibiotic era where common infections become untreatable. The implications of this crisis are profound, affecting medical procedures, public health, and global economies.
Key statistics highlighting the severity of the issue include:
- 700,000 deaths annually are attributed to antibiotic-resistant infections.
- By 2050, this number could rise to 10 million deaths per year if no action is taken.
- The economic impact of antibiotic resistance is estimated to be $100 trillion by 2050.
Addressing this crisis requires a multifaceted approach, including the development of new antibiotics, improved stewardship of existing antibiotics, and global cooperation to combat the spread of resistant bacteria.
The Legacy of Alexander Fleming and the Future of Antibiotics
The legacy of Alexander Fleming extends far beyond his discovery of penicillin. His work laid the foundation for modern antibiotic therapy and inspired generations of scientists to explore the potential of antimicrobial agents. This section reflects on Fleming's enduring impact and the future of antibiotic research.
Fleming's Contributions to Microbiology
Fleming's contributions to microbiology are vast and varied. In addition to his discovery of penicillin, he made significant advancements in the understanding of bacterial infections and the body's immune response. His work on lysozyme provided insights into the body's natural defense mechanisms, while his research on antibacterial agents paved the way for the development of numerous life-saving drugs.
Fleming's approach to scientific inquiry, characterized by curiosity and keen observation, serves as a model for researchers today. His ability to recognize the potential in seemingly mundane observations, such as a contaminated Petri dish, highlights the importance of curiosity-driven research.
The Future of Antibiotic Research
The ongoing challenge of antibiotic resistance underscores the need for continued innovation in the field of antimicrobial research. Scientists are exploring several avenues to address this crisis, including:
- Development of New Antibiotics: Research efforts are focused on discovering novel antibiotics with unique mechanisms of action.
- Alternative Therapies: Approaches such as phage therapy, which uses viruses to target bacteria, are being investigated.
- Antibiotic Stewardship: Programs aimed at promoting the responsible use of antibiotics to preserve their efficacy.
The future of antibiotic research holds promise, with advancements in technology and a deeper understanding of bacterial biology driving innovation. However, the lessons of the past, embodied in Fleming's warnings about resistance, must guide these efforts to ensure the continued effectiveness of antibiotics.
In the final part of this article, we will explore the broader implications of Fleming's discovery, its impact on society, and the ongoing efforts to honor his legacy. Stay tuned for a comprehensive conclusion to our exploration of Alexander Fleming and the antibiotic revolution.
The Societal Impact of Penicillin and the Antibiotic Era
The discovery of penicillin by Alexander Fleming not only revolutionized medicine but also had profound societal implications. This section explores how the antibiotic era transformed public health, extended life expectancy, and reshaped medical practices worldwide.
Transforming Public Health and Life Expectancy
Before the antibiotic era, infectious diseases were the leading cause of death globally. Conditions like pneumonia, tuberculosis, and sepsis claimed millions of lives annually. The introduction of penicillin dramatically altered this landscape:
- Reduction in Mortality Rates: Penicillin's effectiveness against bacterial infections led to a 20-30% decrease in mortality rates from treatable diseases within a decade of its widespread use.
- Increased Life Expectancy: Global life expectancy rose by 8-10 years in the mid-20th century, with antibiotics playing a crucial role in this improvement.
- Decline in Child Mortality: Infant mortality rates dropped significantly as antibiotics became available to treat childhood infections.
These changes had far-reaching economic and social consequences, allowing populations to grow healthier and more productive.
Changing Medical Practices and Hospital Care
The availability of effective antibiotics transformed medical practices in numerous ways:
- Surgical Advancements: Complex surgeries that were previously too risky due to infection concerns became viable. Organ transplants, joint replacements, and cardiac surgeries all benefited from antibiotic prophylaxis.
- Hospital Infection Control: The ability to treat infections reduced the fear of hospital-acquired infections, making medical facilities safer for patients.
- Chronic Disease Management: Patients with chronic conditions like diabetes or cancer, who are more susceptible to infections, experienced improved outcomes.
The antibiotic era fundamentally changed how doctors approached patient care, shifting from reactive treatment of infections to preventive measures and more aggressive medical interventions.
Alexander Fleming's Enduring Influence on Science and Medicine
Beyond his scientific discoveries, Alexander Fleming's approach to research and his personal philosophy continue to inspire scientists and medical professionals today. This section examines his lasting influence on the scientific community and medical education.
Fleming's Scientific Method and Legacy
Fleming's discovery of penicillin exemplifies several key principles that remain fundamental to scientific research:
- Observational Skills: His ability to notice the antibacterial effect in a contaminated Petri dish highlights the importance of keen observation in scientific discovery.
- Interdisciplinary Approach: Fleming's work bridged microbiology, chemistry, and medicine, demonstrating the value of interdisciplinary research.
- Persistence: Despite initial skepticism about penicillin's potential, Fleming continued his research, eventually leading to its development as a life-saving drug.
These principles continue to guide scientific inquiry and innovation in the 21st century.
Inspiring Future Generations of Scientists
Fleming's story has become a cornerstone in medical education, inspiring countless students to pursue careers in microbiology and pharmaceutical research. His life and work demonstrate:
- The potential for groundbreaking discoveries to come from unexpected sources
- The importance of collaboration in scientific progress
- The ethical responsibility of scientists to consider the long-term implications of their discoveries
Many modern researchers cite Fleming as a key influence in their decision to study infectious diseases and antibiotic development.
Preserving Fleming's Legacy: Museums, Awards, and Commemorations
The global recognition of Alexander Fleming's contributions has led to numerous commemorations and institutions dedicated to preserving his legacy. This section explores how his work continues to be honored worldwide.
Museums and Historical Sites
Several institutions around the world celebrate Fleming's achievements:
- The Alexander Fleming Laboratory Museum in London, located at St. Mary's Hospital where penicillin was discovered, preserves his original laboratory and artifacts.
- The Fleming Museum in Scotland showcases his early life and scientific journey.
- Exhibits at the Science Museum in London and the Smithsonian Institution in Washington, D.C. feature penicillin's development and impact.
These museums serve as educational resources, helping the public understand the significance of antibiotic discovery and the ongoing challenges in infectious disease treatment.
Scientific Awards and Honors
Fleming's name has become synonymous with scientific excellence in microbiology:
- The Fleming Prize, awarded by the Microbiology Society, recognizes outstanding research in microbiology.
- Numerous universities have established Fleming Scholarships for students pursuing studies in medical research.
- His portrait appears on banknotes and stamps in several countries, commemorating his contributions to science.
These honors ensure that Fleming's legacy continues to inspire new generations of scientists and medical professionals.
Lessons from Fleming's Discovery: Addressing Modern Challenges
The story of penicillin offers valuable lessons for addressing contemporary challenges in medicine and public health. This section examines how Fleming's experiences can inform our approach to current and future health crises.
Applying Fleming's Principles to Antibiotic Resistance
Fleming's early warnings about antibiotic resistance provide crucial insights for combating this modern crisis:
- Responsible Antibiotic Use: Fleming's observations about resistance development underscore the need for antibiotic stewardship programs in hospitals and communities.
- Investment in Research: The prolonged period between penicillin's discovery and its mass production highlights the importance of sustained research funding.
- Global Cooperation: The international collaboration that enabled penicillin's development serves as a model for addressing global health challenges.
These principles are particularly relevant as we face the growing threat of antimicrobial resistance, which the WHO has identified as one of the top 10 global public health threats.
Innovation in Antimicrobial Development
The penicillin story demonstrates the potential for innovative solutions to emerge from unexpected sources. Modern approaches to antimicrobial development include:
- CRISPR Technology: Gene-editing tools that could target bacterial DNA with precision.
- Phage Therapy: Using bacteriophages (viruses that infect bacteria) as an alternative to traditional antibiotics.
- Antimicrobial Peptides: Naturally occurring compounds that show promise in combating resistant bacteria.
These innovative approaches, inspired by the spirit of Fleming's discovery, offer hope in the fight against antibiotic-resistant infections.
Conclusion: The Lasting Impact of Alexander Fleming's Discovery
The discovery of penicillin by Alexander Fleming in 1928 stands as one of the most significant milestones in medical history. This accidental yet revolutionary finding transformed the treatment of bacterial infections, saved countless lives, and laid the foundation for modern antibiotic therapy. As we reflect on Fleming's contributions, several key takeaways emerge:
- Scientific Serendipity: Fleming's discovery reminds us that groundbreaking innovations often come from unexpected observations and curiosity-driven research.
- Collaborative Progress: The development of penicillin into a viable medical treatment required the combined efforts of Fleming, Florey, Chain, and many others, demonstrating the power of scientific collaboration.
- Global Health Transformation: Penicillin's introduction marked the beginning of the antibiotic era, dramatically reducing mortality rates and extending life expectancy worldwide.
- Ongoing Challenges: Fleming's early warnings about antibiotic resistance highlight the need for responsible antibiotic use and continued research into new treatments.
- Enduring Legacy: From museums to scientific awards, Fleming's contributions continue to inspire and educate future generations of scientists and medical professionals.
As we face the challenges of antibiotic resistance and emerging infectious diseases, the story of Alexander Fleming and penicillin serves as both a source of inspiration and a cautionary tale. It reminds us of the transformative power of scientific discovery while underscoring the importance of responsible innovation and global cooperation in addressing health crises. The antibiotic revolution sparked by Fleming's discovery continues to shape modern medicine, and his legacy endures as a testament to the profound impact that a single scientific breakthrough can have on humanity.
In an era where the threat of antibiotic-resistant bacteria looms large, the lessons from Fleming's discovery are more relevant than ever. By embracing the spirit of curiosity, collaboration, and responsible innovation that characterized his work, we can honor his legacy while forging new paths in the ongoing battle against infectious diseases. The story of Alexander Fleming and penicillin is not just a chapter in medical history—it is a continuing narrative that challenges and inspires us to push the boundaries of scientific discovery for the betterment of global health.
Félix d'Herelle: The Pioneer of Bacteriophage Therapy
The world of microbiology is adorned with a plethora of brilliant minds who have left indelible marks on the scientific landscape. Among these towering figures stands Félix d'Herelle, a self-taught scientist whose groundbreaking work led to the discovery of bacteriophages—viruses that infect and destroy bacteria. Through his pioneering efforts, d'Herelle laid the foundation for bacteriophage therapy, offering a glimmer of hope in an era before the widespread use of antibiotics.
Early Life and the Beginnings of a Scientific Journey
Félix d'Herelle was born on April 25, 1873, in Montreal, Canada, to a well-traveled French family. As a young boy, d'Herelle exhibited an intense curiosity about the natural world, a trait that would define his career. Unlike many of his scientific contemporaries, d'Herelle never pursued formal higher education. Instead, he voraciously read scientific literature and sought hands-on experience, leading to a unique blend of enthusiasm and prowess in scientific inquiry.
In 1899, d'Herelle's journey took him to Guatemala, where he began to experiment in earnest. There, he brewed beer and studied fermentation, igniting his interest in the microbial world. These formative years were characterized by a relentless pursuit of knowledge outside conventional academic channels, an approach that would shape d'Herelle's scientific endeavors and open-minded approach to research.
The Discovery of Bacteriophages
D'Herelle's most significant breakthrough came after he joined the Pasteur Institute in Paris in 1911. There, he focused on understanding dysentery and cholera, which were rampant in France at the time. His investigations into these bacterial infections led to one of the most significant discoveries in microbiology—the existence of bacteriophages.
In 1917, while conducting research on soldiers suffering from dysentery, d'Herelle observed that certain microscopic entities could lyse or destroy bacterial cultures. He documented these observations with meticulous detail, proposing the existence of "invisible antagonists" of bacteria, which he later named bacteriophages. These viruses were found to be specific to certain bacteria, raising the possibility of using them as therapeutic agents.
Although the discovery was met with skepticism, d'Herelle's work steadily gained traction. His meticulous documentation and persistent advocacy for bacteriophage therapy paved the way for its adoption in treating bacterial infections, offering a novel approach that was especially crucial before the advent of antibiotics.
Bacteriophage Therapy: Hope before Antibiotics
The discovery of bacteriophages provided an alternative to treating bacterial infections, long before the discovery of penicillin in 1928 by Alexander Fleming. D'Herelle was a fervent proponent of using bacteriophages in therapeutic settings to combat infectious diseases. His conviction in their effectiveness led to clinical trials and widespread use in treating ailments such as dysentery, cholera, and even typhoid fever in the early 20th century.
However, the path to acceptance was not without its challenges. The medical community was polarized, divided between skepticism and curiosity over d'Herelle’s claims. Despite this, d'Herelle's research laid the groundwork for future therapeutic use, influencing studies on bacteriophage properties, specificity, and effectiveness in clinical settings.
His work gained particular prominence in regions such as Eastern Europe and the former Soviet Union, where bacteriophage therapy continues to be utilized today. D'Herelle's advocacy and scientific contributions helped establish a legacy that remains relevant in modern microbiology, especially in the context of rising antibiotic resistance.
The Legacy of Innovation and Dedication
Félix d'Herelle's contributions extend beyond the discovery of bacteriophages. His enduring impact is rooted in his innovative spirit and unwavering dedication to scientific inquiry. He was a pioneer who bridged the gap between traditional academic environments and practical, problem-solving scientific approaches. With a career that defied conventional academic paths, d'Herelle embodied the essence of a self-taught scientist confronting the challenges of his time with diligence and ingenuity.
As the world continues to grapple with antibiotic resistance, d'Herelle's work is undergoing a renaissance, with bacteriophage therapy emerging as a promising alternative or complement to antibiotics. His legacy is a testament to the transformative power of scientific curiosity and perseverance, influencing modern research, medical treatments, and the broader field of microbiology.
In our next installment, we delve deeper into d'Herelle's later life, exploring his global influence, the broader impact of his discoveries in the scientific community, and the enduring relevance of bacteriophage therapy in contemporary medicine. Stay tuned as we continue to uncover the fascinating journey of Félix d'Herelle, a visionary who dared to look beyond the visible world and changed the course of medical science.
Global Impact and Collaborations
After his monumental discovery of bacteriophages, Félix d'Herelle began to garner attention from various corners of the globe. His work attracted the interest of scientists and medical professionals eager to explore this novel concept of viral therapy against bacterial infections. D'Herelle's career soon took on an international dimension, marked by travels and collaborations that would extend the reach of his innovative ideas and solidify his reputation as a pioneer in microbiology.
In the 1920s, d'Herelle's research took him to multiple continents. He worked extensively in countries such as India and Egypt, where bacterial infections like cholera were prevalent. His interventions demonstrated the potential of bacteriophage therapy to alleviate public health crises, as he successfully applied his methods to real-world applications. These international ventures not only spread the knowledge of bacteriophages but also highlighted the importance of cross-cultural scientific exchanges in the fight against infectious diseases.
During this period, d'Herelle also collaborated with Georgian bacteriologist George Eliava. This partnership, which began at the Pasteur Institute, led to the establishment of the Eliava Institute in Tbilisi, Georgia—a major center for bacteriophage research to this day. The collaboration between d'Herelle and Eliava was more than a professional alliance; it was a fusion of ideas and aspirations toward advancing the therapeutic potential of bacteriophages, paving the way for ongoing research in the field.
Challenges and Controversies
Despite the promising applications of bacteriophages, Félix d'Herelle’s journey was not devoid of challenges. The scientific community met his ideas with a mix of intrigue and skepticism. In the early 20th century, virology was still in its nascent stages, and the mechanisms behind bacteriophages were not fully understood. This lack of comprehension led to controversies about their efficacy and safety, hindering widespread acceptance.
Moreover, the emergence of antibiotics in the late 1930s and 1940s overshadowed bacteriophage therapy. When penicillin and other antibiotics proved remarkably effective against a broad spectrum of bacteria, interest and investment in bacteriophage research waned. The focus shifted towards antibiotic solutions, with bacteriophage therapy being largely sidelined in Western medicine.
Nevertheless, d'Herelle remained steadfast in his belief in the potential of bacteriophages. He continued to advocate for their use, particularly in regions where antibiotics were scarce or ineffective due to resistance. His unwavering commitment to his research in the face of adversity underscored his resolute character and dedication to advancing medical science.
Enduring Influence and Modern Resurgence
In an ironic twist of fate, the scientific community’s initial skepticism of Félix d'Herelle’s discoveries is being re-evaluated in the context of the modern-day challenge of antibiotic resistance. As bacteria evolve and become resistant to existing antibiotics, the global health community is revisiting the potential of bacteriophage therapy as a viable alternative or complementary treatment.
Countries such as Poland and Russia, where research into bacteriophages has continued uninterrupted, are at the forefront of this resurgence. These nations have amassed decades of clinical experience utilizing phage therapy, data that is now invaluable as the world seeks solutions to combat resistant bacterial strains.
Modern advancements in molecular biology and genetic engineering further enhance the potential of bacteriophage therapy. Current research efforts are focused on engineering phages to improve their therapeutic efficacy, targeting specificity, and overcoming hurdles such as bacterial resistance. This new era of phage research is breathing life into d'Herelle’s early 20th-century visions, blending classical microbiology with cutting-edge biotechnology.
The Timeless Vision of Félix d'Herelle
As the renaissance of bacteriophage therapy unfolds, Félix d'Herelle’s influence resonates more profoundly than ever. He was a trailblazer who, through perseverance and ingenuity, advocated for a path less taken in the realm of medical science. His ability to envisage solutions beyond the scope of current knowledge remains a hallmark of innovative thinking in scientific endeavors.
D'Herelle’s legacy exemplifies the power of pursuing scientific understanding with tenacity and open-mindedness. His contributions continue to inspire new generations of researchers committed to combating the persistent and ever-evolving challenges posed by infectious diseases.
In the final segment of this series, we will delve into the personal aspects of d'Herelle's life, exploring his character, motivations, and the lasting impact of his work on contemporary scientific research and healthcare. Join us as we conclude our exploration of Félix d'Herelle, an awe-inspiring leader whose visionary insights continue to shape the future of microbiology and therapeutic innovation.
The Personal Side of a Scientific Trailblazer
Beyond his groundbreaking scientific contributions, Félix d'Herelle was a man of remarkable character and intriguing personal dimensions. A self-taught polymath with an unconventional career, d'Herelle was driven by an unyielding curiosity and a deep-seated passion for advancing medical science. His journey was marked by both triumphs and tribulations, underscoring a profound dedication to the art of discovery.
D'Herelle was known for his relentless pursuit of understanding. His work was characterized by an unwavering intensity and a hands-on approach to experimentation. Despite lacking formal academic qualifications, he harbored an innate scientific intuition that allowed him to conceptualize and execute complex research endeavors. This commitment to self-directed learning and exploration was emblematic of d'Herelle’s innovative spirit.
His collaborations with scientists like George Eliava also reflect his openness and willingness to share ideas. By forging international connections, d'Herelle transcended the geographic and cultural barriers of his time, building a network of like-minded researchers who supported and expanded upon his work. These collaborations not only enriched his own research but also fostered a collaborative ethos within the scientific community.
Legacy and the Human Element
Félix d'Herelle's legacy is not merely confined to his scientific achievements. His life and work embody the essential qualities of perseverance, intellectual curiosity, and a profound belief in the potential of scientific inquiry to resolve pressing health challenges. D'Herelle's legacy is an inspiring testament to the power of human dedication when guided by a compelling vision.
He was a visionary who dared to challenge the status quo and explore uncharted territories in microbiology. In doing so, d'Herelle helped usher in a new era of understanding and therapeutic possibilities. His pioneering spirit continues to inspire modern researchers who face the daunting task of overcoming contemporary challenges such as antibiotic resistance and emerging infectious diseases.
In recent years, the relevance of d'Herelle's work has been further accentuated by its adaptation to modern contexts. The exploration of bacteriophage therapy as a countermeasure to antibiotic resistance has reignited interest in d'Herelle’s earlier insights, illustrating the enduring nature of his contributions. As a symbol of the continuous journey of scientific progress, his legacy persists in influencing research, shaping medical practices, and inspiring future scientific endeavors.
The Continuing Impact of a Visionary
Today, research institutions across the globe are revisiting bacteriophage therapy and investing in its potential development. The renewed interest highlights the timeless insight Félix d'Herelle possessed, recognizing bacteriophages as significant players in the battle against bacterial infections. His early 20th-century work is integral to the resurgence of phage therapy as a promising biological tool in modern medicine.
Modern bacteriophage research extends to various fields, including agriculture, environmental science, and biotechnology, where phages are explored for their ability to target and neutralize specific bacteria. This multidisciplinary application underscores the versatility and potential of d'Herelle’s discovery, demonstrating its broad relevance beyond traditional medical paradigms.
Furthermore, the personal attributes of innovation and resilience that characterized d'Herelle’s life serve as a beacon for aspiring scientists. His ability to transform challenges into opportunities and maintain a steadfast focus on his research goals, despite skepticism and setbacks, offers a timeless lesson on the importance of perseverance and adaptability in scientific pursuits.
Conclusion: Celebrating a Life of Discovery
Félix d'Herelle’s life journey, punctuated by scientific breakthroughs and unwavering dedication, is a powerful narrative of discovery and vision. His pioneering work in bacteriophage research and therapy laid a foundational stone for modern microbiology, contributing a transformative approach to combating bacterial infections. As the world continuously battles the complexities of emerging health threats, his work remains a critical reference point.
Celebrating Félix d'Herelle is to celebrate the spirit of innovation and the relentless pursuit of knowledge that drives scientific progress forward. As we stand on the shoulders of such trailblazers, it is their legacy that encourages new generations to pursue the unknown, challenge established norms, and strive for advancements that ultimately improve human lives.
The story of Félix d'Herelle is as much about the science he championed as it is about the indomitable human spirit. His legacy endures as a symbol of what can be achieved when curiosity, determination, and a vision for the future intersect to illuminate paths previously unexplored. It is this legacy that continues to inspire and will remain vital as science reaches for new frontiers in the years to come.