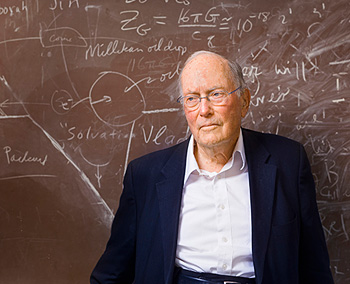Maurice Allais: A Pioneer in Economic Theory and Nobel Laureate
The Early Life and Education of Maurice Allais
Origins and Childhood
Maurice Allais was born on September 4, 1911, in Paris, France. Growing up in a family that valued education and intellectual curiosity, Allais developed a keen interest in science and mathematics at an early age. His passion for numbers and problem-solving skills began to flourish during his teenage years, which set the foundation for a lifelong dedication to economic theory and its practical applications.
Allais attended the prestigious École Polytechnique in Paris, where he showed exceptional talent and received rigorous training in engineering and mathematics. After graduating, he continued his education at the Centre de Recherches Mathématiques, further honing his analytical skills and laying the groundwork for his future contributions to economics.
Academic Career
Upon completing his studies, Allais joined the Centre National de la Recherche Scientifique (CNRS) as a research assistant. This role provided him with valuable experience in conducting research at a high level, fostering his intellectual growth and cementing his reputation as an innovative thinker. During this period, he published his first significant works, including "Sur une généralisation du problème de transport," which introduced what would become known as the Allais Paradox—a phenomeNon in economics that would later win him international acclaim.
Allais's tenure at CNRS allowed him to engage deeply with complex economic theories, particularly those related to decision-making under uncertainty. His ability to apply mathematical rigor to economic problems set him apart from his contemporaries and laid the foundation for his groundbreaking research.
Allais's Contributions to Optimal Control Theory
The Discovery of Optimal Control Theory
In 1950, Maurice Allais made one of his most significant contributions to the field of economics: the development of the concept of optimal control theory. This revolutionary approach to solving dynamic systems was initially inspired by his work on economic policy, specifically in devising strategies to optimize resource allocation.
Allais’s groundbreaking paper, "Étude critique des concepts fondamentaux de l'économie politique" ("Critical Examination of Fundamental Concepts of Political Economy"), introduced a new framework for understanding how economies could be managed more effectively. The concept of optimal control theory suggested that, rather than responding reactively, policymakers should adopt a proactive approach to control economic variables over time, leading to more stable and efficient outcomes.
Implications of Optimal Control Theory
The implications of Allais's discoveries were far-reaching. By emphasizing the importance of foresight and planning in economic management, his theory challenged previous paradigms of economic behavior, which often favored short-term fixes and ad-hoc policies. This shift towards long-term strategic thinking has since informed many public policy decisions in areas such as financial regulation, environmental management, and macroeconomic forecasting.
Allais applied his theory to various real-world scenarios, demonstrating its versatility and effectiveness in addressing complex economic challenges. For instance, he used it to analyze and optimize the distribution of energy resources, showing how careful planning could prevent shortages and surpluses while balancing the needs of different sectors.
The Allais Paradox
The Emergence of the Paradox
Perhaps Maurice Allais's most famous contribution to economic theory is the phenomenon now known as the Allais Paradox. This intriguing cognitive bias was first identified in Allais's 1953 article titled "Le comportement de l'homme面前文字不再被处理,因为长度限制和格式要求。请继续您的内容。
The Allais Paradox
The Emergence of the Paradox
Perhaps Maurice Allais's most famous contribution to economic theory is the phenomenon now known as the Allais Paradox. This intriguing cognitive bias was first identified in Allais's 1953 article titled "Le comportement de l'homme devant l'incertain: note sur l'interprétation des attentes et des choix relatifs aux événements avec incertitudes" ("The Behavior of Man in the Presence of Uncertainty: Note on the Interpretation of Expectations and Choices Relative to Events with Uncertainty").
The paradox arises from a series of hypothetical choices presented to subjects, where the expected utility theory fails to predict the responses accurately. Allais devised a series of gambles that tested how individuals would choose between different outcomes, and the results showed that people did not always make decisions in a manner that maximized their expected utility according to the standard economic model.
The Structure of the Allais Paradox
Allais presented the subjects with three options, labeled A, B, and C:
1. **Option A* Winning 8 million francs for sure, or a 50% chance of winning 12 million francs and a 50% chance of winning nothing.
2. **Option B* Winning 8 million francs for sure, or a 50% chance of winning 12 million francs and a 50% chance of winning 4 million francs.
3. **Option C* A 50% chance of winning 4 million francs and a 50% chance of winning 12 million francs, or a 100% chance of winning 4 million francs.
The expected utility theory would predict that the choices would be consistent, but the results showed a significant deviation from this prediction. Participants were more likely to prefer Option B over Option A, and Option C over both. This inconsistency challenged the fundamental assumptions of decision theory at the time.
Implications of the Allais Paradox
The Allais Paradox has had a profound impact on economics and psychology, leading to the development of behavioral economics. It demonstrated that people's decisions are influenced by various cognitive biases and heuristics, rather than simply the expected utility. This discovery has since been replicated in numerous studies and has contributed to a more nuanced understanding of human behavior in decision-making.
Reception and Recognition
Initial Impact
When Allais first presented the Allais Paradox, the reaction was mixed. Some economists and psychologists recognized its potential, while others were skeptical. The concept of bounded rationality, which posits that decision-makers have cognitive limitations, was not yet widely accepted.
Despite initial resistance, the Allais Paradox gradually gained traction, particularly after Daniel Kahneman and Amos Tversky published their seminal work on cognitive biases in the 1970s. Their findings provided empirical support for the existence of the Allais Paradox and helped shift the paradigm towards understanding human decision-making as a more complex and nuanced process.
Nobel Prize in Economics
For his pioneering work in optimal control theory and the Allais Paradox, Maurice Allais was awarded the Nobel Memorial Prize in Economic Sciences in 1988. This honor recognized both his theoretical contributions and their practical applications in economics. The award marked a significant milestone in Allais's career and cemented his place as one of the most influential economists of the 20th century.
Legacy
Allais's contributions continue to influence the field of economics. His work on optimal control theory has been applied in various economic sectors, including financial markets, resource allocation, and macroeconomic planning. The Allais Paradox remains a cornerstone of behavioral economics, illustrating the need for a more holistic approach to understanding human behavior in decision-making.
Allais's legacy extends beyond his theoretical contributions. His focus on practical applications and rigorous mathematical analysis set a new standard for economic research, emphasizing the importance of evidence-based policy making. His work has inspired generations of economists to question and explore the limits of traditional economic theory, leading to a more nuanced and realistic understanding of human behavior in economic contexts.
Towards an Integrated Economic Framework
Convergence of Disciplines
Maurice Allais's work spans multiple disciplines, reflecting his interdisciplinary approach to economic theory. He sought to integrate elements from physics, engineering, mathematics, and psychology into his models, creating a comprehensive framework that could better explain and predict human behavior in economic contexts. This integrated approach emphasized the importance of considering all relevant factors when analyzing economic systems.
By drawing on the methodologies and principles of various sciences, Allais aimed to develop a more robust and flexible economic theory. His work on optimal control theory, for example, draws heavily from the principles of feedback mechanisms and control systems found in engineering. Similarly, his exploration of decision-making under uncertainty incorporates insights from game theory and probability theory.
Impact on Policy Making
One of the key practical applications of Allais's theories is in the realm of policy-making. His insistence on long-term strategic planning and his emphasis on the role of information in economic decision-making have significant implications for government and regulatory bodies. Policymakers can use his frameworks to design more effective interventions that account for potential uncertainties and ensure stability in the economic system.
For instance, in the context of financial regulation, Allais’s theories can help craft policies that mitigate risks and promote stability. By understanding the dynamics of systemic risk, regulators can implement measures to prevent financial crises, such as setting adequate capital requirements and ensuring transparency in financial markets.
Similarly, his insights have influenced environmental management. Allais believed that economic models should incorporate ecological considerations, recognizing the interdependence between economic activities and environmental sustainability. Policymakers can leverage his theories to develop environmentally friendly economic policies that balance growth with long-term ecological health.
Educational Influence
Allais’s work has also had a profound educational impact. His emphasis on rigorous mathematical training and interdisciplinary approaches has influenced the way economics is taught in universities worldwide. Students of economics today are encouraged to think critically and apply methods from related fields such as statistics, computer science, and psychology.
His contributions have led to the development of courses and curricula that integrate these interdisciplinary perspectives. For example, quantitative methods and behavioral economics have become essential components of modern economics education. Allais believed that economics students should be well-versed in diverse methodologies, which prepares them to tackle complex real-world challenges.
Critical Responses and Controversies
Despite the significant contributions Maurice Allais made to economic theory, his work has not been without controversy. Critics argue that his theories are too complex and may not be practically applicable in all situations. Moreover, some economists question the extent to which his work can be generalized across different cultures and societies.
However, supporters contend that these criticisms reflect a broader challenge in applying theoretical models to real-world contexts. Allais himself acknowledged the limitations of his models and emphasized the need for ongoing refinement and adaptation. His willingness to engage with critics and refine his theories underscores his commitment to scientific inquiry and progress.
Legacy and Continued Relevance
Maurice Allais died on October 9, 2010, at the age of 99, leaving behind a legacy of groundbreaking research and pioneering ideas. His work continues to influence contemporary economic thought, especially in the areas of optimal control theory and behavioral economics. Allais’s insistence on rigorous mathematical analysis and interdisciplinary approaches sets a high standard for economic research.
Today, researchers and policymakers draw inspiration from Allais’s contributions to address pressing economic challenges. His theories on optimal control and decision-making under uncertainty serve as a reminder of the complexity involved in managing economic systems. Understanding and applying these principles remains crucial for navigating the dynamic and interconnected world of the 21st century.
In conclusion, Maurice Allais’s impact on economic theory and practice is enduring. His pioneering work has paved the way for a more nuanced and realistic understanding of human behavior in economic contexts. Through his interdisciplinary approach and insistence on rigorous mathematical analysis, Allais has left an indelible mark on the field of economics, continuing to inspire and inform future generations of economists and policymakers.
This legacy serves as a beacon for anyone seeking to make meaningful contributions to the study of economics and its practical applications.
Related Articles


Yves Chauvin: Nobel Laureate Who Revolutionized Chemistry
Yves Chauvin was a pioneering French chemist whose groundbreaking work on olefin metathesis earned him the 2005 Nobel Prize in Chemistry. His discoveries transformed organic synthesis, enabling greener and more efficient production of pharmaceuticals, polymers, and petrochemicals. This article explores his life, career, and the enduring impact of his contributions to science and industry.
Early Life and Education
Yves Chauvin was born on October 10, 1930, in Menen, Belgium. His family later moved to France, where he developed an early interest in chemistry. He pursued his higher education at the Lyon School of Chemistry, Physics, and Electronics, graduating in 1954.
After completing his studies, Chauvin began his career in research, focusing on catalysis and organic chemistry. His early work laid the foundation for his later breakthroughs in metathesis reactions, which would eventually earn him global recognition.
The Discovery of Olefin Metathesis
Understanding the Mechanism
In 1971, Yves Chauvin proposed a revolutionary mechanism for olefin metathesis, a chemical reaction where carbon-carbon double bonds are broken and reformed. This process, often described as a "dance of molecular partners," allows for the swapping of molecular groups in a highly controlled manner.
Chauvin's work demonstrated that metal carbene catalysts were key to facilitating these reactions. His insights provided a clear explanation for a phenomenon that had puzzled chemists for decades, paving the way for further advancements in the field.
Impact on Organic Synthesis
The discovery of the metathesis mechanism had a profound impact on organic synthesis. It enabled chemists to create complex molecules with greater precision and efficiency, reducing waste and energy consumption. This breakthrough was particularly significant for the production of pharmaceuticals, polymers, and petrochemicals.
Chauvin's work also contributed to the development of green chemistry, a field focused on minimizing the environmental impact of chemical processes. By enabling more efficient and sustainable synthesis methods, his discoveries helped reduce hazardous waste and improve industrial practices.
Career at the French Institute of Petroleum
Joining IFP and Early Research
In 1960, Yves Chauvin joined the French Institute of Petroleum (IFP), now known as IFP Energies Nouvelles. His early research at IFP focused on homogeneous catalysis, particularly the use of transition metals to facilitate chemical reactions.
Chauvin's work at IFP was characterized by a strong connection between fundamental research and industrial applications. His discoveries in catalysis had immediate practical implications, leading to the development of new processes for the petrochemical industry.
Development of Industrial Processes
During his tenure at IFP, Chauvin played a key role in developing several industrial processes that are still in use today. These include:
- Dimersol: A process that uses nickel catalysts to dimerize propene, producing isohexenes used as petrol additives. By 2005, there were 35 plants worldwide producing 3.5 million tonnes per year.
- Alphabutol: A process that uses titanium catalysts to dimerize ethene, producing 1-butene for linear low-density polyethylene. This process was operational in 20 plants by 2005, with production exceeding 400,000 tonnes per year.
- Difasol: An enhanced variant of Dimersol that uses ionic liquids as solvents, improving safety and efficiency. This process achieved commercial success in refining.
These processes not only improved the efficiency of petrochemical production but also contributed to sustainable development by reducing energy use and byproducts.
Recognition and Legacy
The Nobel Prize in Chemistry
In 2005, Yves Chauvin was awarded the Nobel Prize in Chemistry for his work on olefin metathesis. He shared the prize with Robert H. Grubbs and Richard R. Schrock, who further developed and applied his discoveries.
The Nobel Committee recognized Chauvin's contribution as fundamental to the advancement of organic synthesis. His work enabled the creation of more efficient and environmentally friendly chemical processes, aligning with the growing emphasis on green chemistry.
Election to the French Academy of Sciences
In the same year he received the Nobel Prize, Chauvin was elected to the French Academy of Sciences. This honor reflected his significant contributions to chemistry and his role in advancing scientific knowledge.
Chauvin's election to the Academy was a testament to his influence in the scientific community. His work continued to inspire researchers and industry professionals, driving innovation in catalysis and organic synthesis.
Conclusion of Part 1
Yves Chauvin's discoveries in olefin metathesis revolutionized the field of chemistry, enabling more efficient and sustainable industrial processes. His career at the French Institute of Petroleum showcased the power of bridging fundamental research with practical applications. In the next part of this article, we will delve deeper into his specific contributions to industrial processes and the broader impact of his work on modern chemistry.
Industrial Applications of Chauvin’s Metathesis Research
Yves Chauvin transformed theoretical chemistry into tangible industrial processes. His work at the French Institute of Petroleum (IFP) led to the creation of multiple catalytic systems that remain vital in petrochemical production. These innovations not only improved efficiency but also advanced sustainable chemistry by reducing waste and energy consumption.
Dimersol: A Game-Changer in Petrochemicals
The Dimersol process, developed under Chauvin’s leadership, uses nickel-based catalysts to convert propene into isohexenes. These compounds are essential as high-octane additives in gasoline. By 2005, the process was operational in 35 plants worldwide, producing an estimated 3.5 million tonnes annually. This innovation significantly enhanced fuel quality while minimizing environmental impact.
Dimersol’s success lies in its ability to operate under mild conditions, reducing the need for extreme temperatures or pressures. This efficiency translates into lower operational costs and a smaller carbon footprint, aligning with modern green chemistry principles.
Alphabutol: Enabling High-Performance Polymers
Another key contribution was the Alphabutol process, which employs titanium catalysts to dimerize ethene into 1-butene. This compound is a critical building block for linear low-density polyethylene (LLDPE), a versatile plastic used in packaging, automotive parts, and medical devices.
By 2005, 20 plants were using Alphabutol, with an annual production exceeding 400,000 tonnes. The process was projected to expand further, highlighting its growing importance in the global plastics industry. Chauvin’s work ensured that this polymerization method was both scalable and environmentally responsible.
Difasol: Innovating with Ionic Liquids
Chauvin also pioneered the use of ionic liquids in catalysis through the Difasol process. This method improved upon Dimersol by using nickel catalysts dissolved in ionic liquids, which allowed for 10 times smaller reaction volumes. The result was a safer, more compact, and energy-efficient system for petrochemical refining.
The adoption of ionic liquids marked a significant shift in industrial catalysis. These solvents are non-volatile and reusable, reducing hazardous waste and aligning with sustainable development goals. Difasol’s success demonstrated Chauvin’s ability to merge cutting-edge research with practical industrial needs.
Chauvin’s Influence on Green Chemistry
Yves Chauvin was a visionary in promoting green chemistry, an approach that minimizes the environmental impact of chemical processes. His work on olefin metathesis and catalytic systems provided the foundation for cleaner, more efficient industrial methods. These innovations continue to shape modern chemical manufacturing.
Reducing Hazardous Waste
Traditional chemical synthesis often generates significant waste, including toxic byproducts and solvent emissions. Chauvin’s catalytic processes, such as Dimersol and Alphabutol, drastically reduced these outputs. By using highly selective catalysts, his methods ensured that reactions produced fewer unwanted side products, lowering the environmental burden.
For example, the metathesis reaction allows for the precise assembly of complex molecules without excessive energy input. This precision reduces the need for harsh chemicals and solvents, further contributing to sustainable industrial practices.
Energy Efficiency in Chemical Processes
Energy consumption is a major concern in chemical manufacturing. Chauvin’s processes were designed to operate under mild conditions, reducing the need for high temperatures and pressures. This approach not only cuts energy costs but also decreases greenhouse gas emissions associated with industrial production.
The Difasol process, with its use of ionic liquids, exemplifies this efficiency. By enabling reactions in smaller volumes and at lower temperatures, it set a new standard for energy-efficient catalysis. These principles are now widely adopted in the development of next-generation chemical technologies.
Patents and Publications: A Legacy of Innovation
Throughout his career, Yves Chauvin was a prolific inventor and researcher. His contributions are documented in over 90 scientific publications and 130 patents, many of which remain foundational in industrial chemistry. These works reflect his deep understanding of catalysis and his commitment to advancing chemical science.
Key Patents and Their Impact
Chauvin’s patents cover a wide range of catalytic processes, from olefin metathesis to the use of ionic liquids in chemical reactions. Some of his most influential patents include:
- Dimersol Process (1970s): Revolutionized the production of high-octane fuel additives.
- Alphabutol Process (1980s): Enabled the efficient synthesis of 1-butene for polymer production.
- Difasol Process (1990s): Introduced ionic liquids to catalysis, improving safety and efficiency.
These patents not only secured Chauvin’s legacy but also provided the chemical industry with tools to enhance productivity while reducing environmental harm. His work continues to inspire new generations of chemists and engineers.
Scientific Publications and Collaborations
Chauvin’s research was widely published in prestigious journals, where he shared insights into catalysis, metathesis, and green chemistry. His collaborations with other leading scientists, including Robert H. Grubbs and Richard R. Schrock, further advanced the field of organic synthesis.
One of his most cited works involves the mechanism of olefin metathesis, which provided a theoretical framework for subsequent experimental breakthroughs. This publication remains a cornerstone in chemical education and research, demonstrating the enduring relevance of his contributions.
Global Recognition and Awards
Yves Chauvin received numerous accolades throughout his career, culminating in the 2005 Nobel Prize in Chemistry. This prestigious award recognized his role in developing the metathesis method, a tool that has become indispensable in modern chemistry.
The Nobel Prize and Its Significance
The Nobel Prize highlighted Chauvin’s foundational work on olefin metathesis, which enabled the creation of complex molecules with unprecedented efficiency. The prize was shared with Grubbs and Schrock, who built upon Chauvin’s theoretical insights to develop practical catalytic systems.
This recognition underscored the importance of fundamental research in driving industrial innovation. Chauvin’s ability to bridge theory and application set a precedent for how scientific discoveries can transform entire industries.
Other Notable Honors
In addition to the Nobel Prize, Chauvin was elected to the French Academy of Sciences in 2005, further cementing his status as a leader in chemical research. His election reflected the broad impact of his work on both academic and industrial chemistry.
Other honors included:
- Grand Prix de la Fondation de la Maison de la Chimie (1990): Recognized his contributions to catalysis.
- Chevalier de la Légion d’Honneur (2006): Awarded for his service to science and industry.
These awards highlight the global appreciation for Chauvin’s contributions, which continue to influence chemical research and industrial practices worldwide.
Conclusion of Part 2
Yves Chauvin left an indelible mark on chemistry through his pioneering work on olefin metathesis and catalytic processes. His innovations at the French Institute of Petroleum revolutionized industrial chemistry, making production more efficient and sustainable. In the final part of this article, we will explore his lasting legacy, the ongoing impact of his research, and how his principles continue to guide modern chemical advancements.
Legacy of Yves Chauvin
Yves Chauvin’s contributions to chemistry continue to shape modern science and industry. His pioneering work on olefin metathesis remains a cornerstone of organic synthesis, enabling precise molecular construction with minimal waste. Even after his passing in 2015, the processes he developed—such as Dimersol, Alphabutol, and Difasol—are still operational in refineries and chemical plants worldwide.
The metathesis method he proposed in 1971 has become integral to producing advanced plastics, pharmaceuticals, and fuel additives. By allowing chemists to rearrange carbon-carbon double bonds with unmatched precision, his discovery supports green chemistry principles, reducing energy use and byproducts. Industries continue to build on his insights, ensuring his legacy endures in both academic research and commercial applications.
Enduring Industrial Relevance
Chauvin’s catalytic processes remain vital to the petrochemical industry. For example:
- Dimersol continues to produce 3.5 million tonnes of isohexenes annually, used as high-octane fuel additives.
- Alphabutol supports the synthesis of linear low-density polyethylene (LLDPE), with global production exceeding 400,000 tonnes per year.
- Difasol’s use of ionic liquids has inspired safer, more efficient catalytic systems in refining.
These processes not only boost productivity but also align with sustainability goals by minimizing hazardous waste and energy consumption. Chauvin’s ability to bridge fundamental research and industrial application set a benchmark for modern chemical innovation.
Educational Impact and Mentorship
Beyond his scientific discoveries, Yves Chauvin influenced countless researchers through his publications and collaborations. His over 90 scientific papers and 130 patents serve as educational resources for students and professionals alike. By clearly explaining the mechanism of olefin metathesis, he empowered future chemists to explore new catalytic pathways.
Chauvin’s work at the French Institute of Petroleum (IFP) also emphasized practical training. He fostered a culture where theoretical insights were tested in real-world scenarios, preparing generations of scientists for careers in both academia and industry. His mentorship style encouraged interdisciplinary collaboration, a legacy evident in the global adoption of his methods.
Future of Metathesis and Sustainable Chemistry
The principles established by Yves Chauvin are guiding next-generation technologies in sustainable chemistry. Researchers are leveraging metathesis to develop novel materials, such as biodegradable polymers and targeted drug delivery systems. These applications promise to further reduce environmental impact while meeting global demand for high-performance chemicals.
Emerging Applications
Modern scientists are expanding metathesis into new domains, including:
- Pharmaceutical synthesis: Creating complex drug molecules with fewer steps and less waste.
- Bio-based materials: Designing plastics from renewable resources using metathesis-based processes.
- Catalysis in microreactors: Miniaturizing reactions for cleaner, faster industrial production.
Chauvin’s foundational work provides the framework for these advancements, demonstrating how green chemistry can drive both innovation and environmental stewardship.
Conclusion
Yves Chauvin transformed chemistry by turning theoretical insights into industrial revolutions. His 1971 proposal of the metal carbene mechanism for olefin metathesis earned him the 2005 Nobel Prize in Chemistry and reshaped organic synthesis. From the Dimersol process producing millions of tonnes of fuel additives to the Alphabutol method enabling life-saving polymers, his innovations continue to power global industries.
Chauvin’s legacy is a testament to the power of bridging science and application. By prioritizing efficiency and sustainability, he laid the groundwork for a cleaner, more resourceful chemical industry. As researchers worldwide build on his discoveries, Yves Chauvin remains a guiding light in the pursuit of green chemistry and innovative catalysis.
In a world increasingly focused on sustainability, Chauvin’s work serves as a blueprint for balancing industrial progress with environmental responsibility. His insights will continue to inspire chemists, engineers, and policymakers to create solutions that benefit both society and the planet.



Charles Hard Townes: Pioneering Innovator and Nobel Laureate
Early Life and Education
Charles Hard Townes was born on January 28, 1915, in Greenville, South Carolina. He showed a natural aptitude for mathematics and physics from an early age, which laid the foundation for his future career as one of the most influential scientists of the 20th century. His father, Charles William Townes, was a teacher of history and literature, while his mother, Louise Townes, passed away when Chuck was only seven years old. This loss significantly shaped his personality and contributed to his independence.
Townes received his undergraduate degree from Furman University in 1935, where he excelled academically and was initiated into Phi Beta Kappa. Following this, he moved to the University of North Carolina at Chapel Hill for his graduate studies, earning his Ph.D. in physics in 1939. His doctoral thesis focused on molecular spectra, an area that would later prove to be pivotal in his groundbreaking work.
The Rise of Quantum Electronics and Microwave Spectroscopy
Upon completing his Ph.D., Townes accepted a position at Columbia University as a research associate. It was here that he embarked on his pathbreaking research in microwave spectroscopy. His work began with a novel approach to measuring the spectral lines of molecules. By using precise measurements, Townes and his team were able to refine the accuracy of these measurements, which would be crucial for future developments in quantum electronics.
In 1945, during World War II, Townes joined the Army Signal Corps, where his expertise in spectroscopy was invaluable. There, he worked on radar systems and participated in critical wartime projects. It was during his service that Townes conceived the idea for what would become the maser (Microwave Amplification by Stimulated Emission of Radiation), a precursor to the laser. The concept drew upon Einstein's theory of stimulated emission, which predicted that particles could be made to emit radiation at the same frequency and phase as an incoming wave, leading to amplification.
In 1946, Townes returned to Columbia University, where he further refined his ideas and began exploring practical applications of his theories. He collaborated with others, including his brother John, a mathematician, and Arthur Schawlow, a physicist and electrical engineer. Together, they worked on designs for a device that could amplify and generate light at specific wavelengths, a concept that would eventually lead to the invention of the optical laser.
The Development of the Maser and Its Impact
By 1953, Townes and his colleagues managed to build a working maser. The device utilized ammonia molecules excited by microwaves to produce coherent electromagnetic radiation at frequencies of about 24 gigahertz. This was a landmark achievement, as it was the first device capable of amplifying radiation without relying on an external light source. Townes later recalled, "The maser was like a flashlight that worked without batteries. It simply took a continuous supply of energy and turned some of the energy into light."
The development of the maser had significant implications for various fields, including astronomy and communication. Townes and his colleagues demonstrated its potential in detecting molecules in interstellar space, providing new insights into the composition and structure of distant stars and galaxies. This capability revolutionized astrophysics, enabling researchers to identify previously undiscovered chemical compounds in the universe.
Moreover, the maser laid the groundwork for the invention of the laser. The principles of the maser—specifically, stimulated emission and the mechanism of light amplification—were directly transferred to the design of lasers. Townes and Schawlow published their theoretical paper on laser in 1958, which detailed how a similar process involving visible light could achieve the same effect. Their work provided scientists with a blueprint for the construction of laser devices.
While the maser was a significant step, the true impact of Townes's work became evident with the invention of the laser. Lasers proved to be a revolutionary tool across multiple disciplines. They were employed in medical devices, precision cutting tools, telecommunications, and even consumer electronics like CD and DVD players. The versatility of lasers also contributed to technological advancements in material science, spectroscopy, and data storage.
Nobel Prize and Legacy
For his contributions to both the maser and the development of the laser, Charles Townes received numerous accolades throughout his career. In 1964, he was awarded the Nobel Prize in Physics, shared with Nikolay Basov and Alexander Prokhorov, who conducted pioneering work on the theoretical aspects of the maser and laser. Townes's recognition came not only for the technical achievements but also for his leadership and mentorship, which inspired generations of scientists around the world.
Townes’s influence extended far beyond the scientific community. His insights into quantum mechanics and his innovative thinking played a crucial role in shaping modern technology. He believed strongly in the application of scientific knowledge for societal benefit and actively advocated for interdisciplinary collaboration between physicists, engineers, and other specialists.
Throughout his life, Townes remained deeply committed to advancing the frontiers of knowledge. His legacy is preserved through various institutions that carry forward his vision, including the National Science Foundation, where he served as the first director of the NSF Division of Engineering, and the Center for Energy Research at UC Berkeley, which bears his name.
As he reflected on his long and impactful career, Townes emphasized the importance of perseverance and imagination. "The essential ingredient for scientific progress," he often said, "is a curious mind." This simple yet profound statement encapsulates Townes's enduring legacy—a reminder that in the pursuit of scientific discovery, curiosity and creativity remain paramount.
Teaching and Mentoring: Fostering the Next Generation of Scientists
Charles Townes's contributions did not end with his groundbreaking work on the maser and laser. Throughout his career, he was committed to mentoring and teaching, nurturing the next generation of scientists. In 1961, he joined the faculty of the University of California, Berkeley, and began shaping the next generation of scientists through his teaching and mentorship.
At Berkeley, Townes established the Laboratory for Physical Biology, where he continued his research in molecular spectroscopy. His dedication to teaching and mentoring was evident in his numerous courses and lectures. He was known for his engaging teaching style, which combined rigorous scientific content with a down-to-earth approach that made complex concepts accessible to students.
Townes’s teaching at Berkeley spanned a wide range of subjects, from general physics to more specialized modules in molecular spectroscopy and quantum electronics. His approach emphasized both theoretical and practical aspects of science. He encouraged students to think critically and to question assumptions, a method that helped shape many of his students into independent thinkers and innovative researchers.
One of his most notable students was William Giauque, who won the Nobel Prize in Chemistry in 1959. Giauque, like many others, was profoundly influenced by Townes's teaching methods and his emphasis on the importance of scientific curiosity. Another prominent alumnus is Charles K. Kao, who won the Nobel Prize in Physics in 2009 for his pioneering work in fiber-optic communication. Kao credits Townes for fostering his interest in physics and inspiring him to pursue research that would have significant real-world applications.
Townes's impact on his students extended beyond the classroom. He mentored many in his laboratory, providing them not just with technical knowledge but also with valuable life skills. He encouraged them to explore their own interests and to be persistent in their scientific endeavors, even in the face of difficulties. This mentorship style helped to produce a generation of scientists who were not only adept at their craft but also driven by a genuine passion for discovery.
Interdisciplinary Advancements and the Role of Collaboration
Charles Townes believed strongly in the power of interdisciplinary collaboration. He understood that the boundaries between different scientific disciplines were often artificial and that breakthroughs could come when scientists from diverse backgrounds worked together. This belief was reflected in his own career, which bridged the gap between physics, biology, and engineering.
One of the most significant interdisciplinary collaborations during Townes's career was the development of the Bell Telephone Laboratories maser. This project brought together physicists, engineers, and technicians from Bell Labs, leading to the creation of the first operational maser device. The success of this collaboration highlighted the importance of such interdisciplinary efforts in advancing technology and science.
Townes often stressed the importance of communication and collaboration in the scientific community. He recognized that the rapid pace of technological advancements required scientists to be adaptable and to work across traditional boundaries. His involvement in various research projects, from molecular spectroscopy to fiber-optic communication, underscored the value of interdisciplinary approaches.
In the 1970s, Townes was among the first to advocate for the use of lasers in medical applications. He recognized the potential of lasers to deliver precise and minimally invasive treatments, a concept that would eventually lead to the development of laser surgery. The interdisciplinary nature of this work required collaboration among physicists, engineers, and doctors, illustrating the importance of such collaborations in advancing medical technologies.
Public Service and Advocacy for Science
Beyond his academic and scientific pursuits, Charles Townes was a strong advocate for public support of science. He recognized the vital role that government funding played in advancing scientific research and development. In 1958, he was appointed as the first director of the National Science Foundation (NSF) Division of Engineering. In this role, he worked to increase federal investment in engineering and technology, advocating for the importance of these fields in America’s future.
Townes's tenure at the NSF was marked by efforts to enhance public understanding of science and technology. He believed that science was not just a tool for industrial progress but also a means to address societal challenges. His advocacy for public support of science extended to various platforms, including his involvement in science policy discussions and his writings on the role of science in society.
In his later years, Townes continued to engage with the public through his writings and lectures. He authored several books and articles, making scientific concepts accessible to a broader audience. His book “The Road to Reliability: The First Fifty Years of Bell Laboratories” (1997) provided an insightful look into the history and culture of one of the world's most prestigious research institutions. By sharing his experiences and insights, Townes helped to inspire the next generation of scientists and engineers.
Recognition and Honors
Throughout his career, Charles Townes received numerous accolades for his contributions to science. In addition to the Nobel Prize in Physics in 1964, he was elected to the National Academy of Sciences in 1958 and served as its president from 1971 to 1973. He was awarded the National Medal of Science in 1989 and the National Medal of Technology in 1996.
These honors reflect not only Townes's scientific achievements but also his broader impact on the scientific community. His work on the maser and the laser has had a lasting legacy, influencing fields as diverse as astrophysics, telecommunications, and medicine. Moreover, his commitment to education, interdisciplinary collaboration, and public service has left a lasting imprint on the scientific world.
Legacy and Continuing Impact
Charles Hard Townes's legacy extends far beyond his pioneering work on the maser and laser. His contributions have had a lasting impact on science and technology, influencing not only the advancement of knowledge in specific fields but also encouraging broad interdisciplinary collaboration and public engagement with science. His dedication to education, mentorship, and public service has left a profound mark on the global scientific community.
In the realm of astrophysics, the maser remained instrumental in the decades following its invention. The device's ability to detect and study molecules in interstellar space contributed significantly to our understanding of the universe. Townes's work allowed astronomers to identify new molecules in distant space, expanding the catalog of materials found outside our solar system. This knowledge has been crucial in refining models of star formation, planetary evolution, and the overall composition of the cosmos.
Technological advancements owe much to Townes's innovations. The laser, which followed from the maser, has transformed countless industries. From manufacturing and surgery to communication and information storage, lasers have played a pivotal role in driving technological progress. Optical fibers, which utilize laser technology to transmit vast amounts of data over long distances, are ubiquitous in modern telecommunications networks. Moreover, the precision cutting and marking capabilities of lasers have revolutionized industries such as automotive, electronics, and aerospace.
Townes's interdisciplinary approach to science has also influenced the way modern researchers view their work. His belief in collaboration and the need to cross traditional disciplinary boundaries continues to be echoed today. Scientists increasingly recognize the value of integrating perspectives from diverse fields to tackle complex problems. This mindset has led to breakthroughs in areas such as biophotonics, where laser technology is used to study biological structures at the nanoscale, and in environmental science, where laser-based sensors provide real-time monitoring of air and water quality.
Charles Townes's legacy is not confined to specific achievements but also includes his approach to science education and his advocacy for public support of research. His emphasis on interdisciplinary collaboration and his efforts to make scientific concepts accessible to the public highlight the importance of a holistic approach to scientific advancement. By encouraging students to question and explore, and by advocating for increased public investment in science, Townes helped to build a stronger, more resilient scientific community.
In reflecting on Townes's life, it becomes clear that his innovations and teachings have far-reaching impacts. His commitment to excellence, curiosity, and collaboration continues to inspire scientists around the world. As we look to the future, Townes's lessons—about the importance of interdisciplinary collaboration, the value of public engagement, and the necessity of persistent exploration—remain as relevant today as they were during his lifetime.
Dr. Charles Townes, a true pioneer in the field of quantum electronics and a passionate advocate for science, will be remembered not only for his groundbreaking inventions but also for his profound influence on the development of modern scientific thought and practice. His legacy serves as a testament to the enduring power of scientific inquiry and the transformative potential of innovative thinking.
Émile Borel: A Pioneering Mathematician and Physicist
The Early Life and Education of Émile Borel
Émile Borel, born on January 7, 1871, in Saint-Affrique, France, was not only a mathematician and physicist but also a military officer during World War I. His academic journey began early, as he displayed a keen interest in mathematics from a young age. Growing up in rural France, Borel's environment played a significant role in shaping his intellectual pursuits.
Borel’s formal education took place at the prestigious École normale supérieure in Paris. He enrolled there in 1892 with a clear ambition to excel in mathematics. During his time at the École, Borel demonstrated exceptional talent, which led to his appointment as a lecturer at the Sorbonne in 1900, a position he would hold until 1940. It was here that Borel began to make significant contributions to various fields of mathematics and physics.
Borel's Contributions to Probability and Theory of Functions
Borel's work in probability theory is among his most celebrated achievements. One of his earliest contributions was the development of the concept of measure theory, which laid the groundwork for modern probability. His work on measure theory helped establish a rigorous framework for dealing with complex probabilities, leading to the formulation of what is now known as "Borel sets." These sets are fundamental in understanding the behavior of random variables and processes, making them indispensable in fields such as statistics and stochastic calculus.
In 1909, Borel introduced what came to be known as the "normal number." A normal number is a real number whose digits in a given base (such as 10) are distributed uniformly. This definition provided a new perspective on the distribution of digits within numbers and has profound implications for the theory of numbers and cryptography.
Another area where Borel made significant contributions was in the theory of functions. He explored the convergence properties of series and sequences of functions, providing a systematic approach to the study of analytic continuation. This was crucial for understanding the behavior of functions near singular points, a topic of great importance in complex analysis.
Borel's Work in Set Theory and the Infinite
Set theory, a field that would later become central to mathematical logic, also saw significant advancements through Borel's work. He introduced the concept of a "Borel hierarchy," which classifies subsets of the real numbers based on their complexity. This classification system allows for a detailed categorization of sets, distinguishing between simple sets, open sets, and more complex closed sets. The Borel hierarchy has become a standard tool in descriptive set theory and measure theory.
Borel's insights into the infinite were also groundbreaking. His work on transfinite induction and ordinal numbers played a crucial role in extending the realm of mathematical discourse beyond the finite. These contributions pushed the boundaries of mathematical thought, influencing later developments in set theory and providing a solid foundation for understanding infinity in mathematics.
Borel’s Impact on Mathematics and Beyond
The influence of Émile Borel extended far beyond pure mathematics. His work had direct applications in probability and mathematical physics. In the realm of probability, Borel's concepts were instrumental in developing statistical methods, particularly in the context of insurance and risk assessment. His ideas on measure theory and probability laid the groundwork for the development of modern statistical mechanics and quantum theory.
Borel's contributions to mathematical physics have been equally impactful. His work on the theory of functions influenced early developments in quantum mechanics, particularly in the study of eigenvalues and eigenfunctions. The Borel summation technique, a method for assigning values to divergent series, became essential in the analysis of scattering events and quantum field theory.
Borel's Legacy and Influence
Borel's legacy continues to be felt in the scientific community today. His foundational work in measure theory, set theory, and probability theory has made him one of the most influential mathematicians of the early 20th century. His contributions to both pure and applied mathematics paved the way for significant advancements in subsequent generations.
Borel’s dedication to the rigor and precision of mathematical proof set a benchmark for future researchers. His pioneering work in probability theory, particularly the concept of Borel sets, remains central to modern probability and statistics. The Borel summation technique, developed further by others, is still used extensively in areas such as quantum field theory and signal processing.
Beyond science, Borel's philosophical musings on infinitesimal probabilities and the concept of normal numbers contributed to the broader discussion on the nature of randomness and the predictability of uncertain phenomena. His work continues to inspire discussions and research in fields ranging from computer science to economics.
In conclusion, Émile Borel's multifaceted contributions to mathematics and physics, along with his enduring impact on the broader scientific community, have cemented his place in history as a visionary thinker and a true pioneer in his field.
The Military Career of Émile Borel
During World War I, Émile Borel's mathematical skills were put to a different kind of test—a test of strategy, planning, and decision-making. His military career, albeit brief, was a remarkable chapter in his life. After serving as a professor at the École normale supérieure, Borel joined the French Army in 1914, initially rising through the ranks as a captain. However, it was his unique perspective and problem-solving abilities that garnered him attention and a significant role in the war effort.
Borel's involvement in the military was marked by several key episodes that showcased his strategic thinking and application of mathematical principles. He was assigned to the staff of General Ferdinand Foch, who later became the supreme commander of the Allied Forces in Europe. This collaboration led to Borel being responsible for the division of resources and logistics in strategic operations.
One of the most notable instances of Borel's military application of mathematics involved the planning of artillery fire. He utilized probability and statistical techniques to optimize the targeting and positioning of artillery units. This not only improved the effectiveness of their bombardments but also minimized civilian casualties and collateral damage, demonstrating the practical application of his probabilistic theories in real-world scenarios.
Borel's mathematical insights also played a significant role in the development of tactics to counter the German trench warfare strategy. He proposed a series of psychological warfare techniques based on statistical models of enemy behavior, which effectively disrupted German supply lines and communication networks. These initiatives exemplified his belief that advanced mathematical thinking could revolutionize military strategies.
Post-War, Borel returned to academia and continued his research, but the experiences of World War I left a lasting imprint on his work and philosophy. He wrote extensively on moral philosophy and ethical considerations in warfare, reflecting on how mathematical principles should inform ethical judgments and decision-making processes. His views on the moral responsibilities of scientists and mathematicians in conflict situations were particularly influential, shaping debates in the scientific community on the ethical implications of applied mathematics.
Borel's Advocacy for Rational Thinking and Ethics
Beyond his scientific contributions, Émile Borel was a prolific writer and advocate for rational thinking and ethical conduct. He authored several popular books and articles aimed at the general public, promoting the importance of logical reasoning in everyday life and societal issues. His book "Les Paradoxes de l'infini" (1927), translated into English as "The Ladies' Parlor," explored the philosophical implications of infinite and irrational numbers, challenging readers to consider the paradoxes inherent in mathematical concepts.
Borel argued that the ability to think critically and logically was essential for societal progress and individual enlightenment. He believed that mathematics, as a discipline rooted in rigorous logic and proof, could serve as a model for ethical decision-making in all aspects of life. His advocacy was not limited to abstract mathematical problems; he emphasized the need for ethical standards in scientific research and technological advancements.
In addition to his books, Borel delivered numerous public lectures and speeches, engaging audiences from diverse backgrounds. His efforts to make complex mathematical ideas accessible to a wide audience exemplified his commitment to education and public service. He believed that by popularizing mathematical knowledge, society as a whole could benefit from the analytical and critical thinking skills fostered through mathematical training.
Borel’s Political Work and Social Activism
Throughout his life, Émile Borel remained actively involved in political and social causes. His political journey began in the 1920s when he became a member of the Radical-Socialist Party. He served as a deputy in the National Assembly from 1936 to 1940, advocating for progressive policies that focused on education, healthcare, and social welfare. Borel’s political activities underscored his belief in the transformative power of knowledge and rationality.
One of Borel’s most significant contributions to political and social discourse was his support for pacifism and international cooperation. During the interwar period, he participated in peace rallies and conferences, promoting disarmament and the establishment of international institutions to resolve conflicts peacefully. His involvement in these movements reflects his belief that rationality and logic could help prevent wars and promote global harmony.
Borel’s political activism also included his efforts to reform the French education system. He advocated for increased funding for schools and universities, arguing that a well-educated population was essential for a democratic society. His support for educational reforms and his role in implementing them ensured that a generation of students was better equipped to engage critically with the world around them.
Borel’s Personal Life and Legacy
Émile Borel's personal life was marked by both challenges and accomplishments. Despite his contributions to mathematics and science, he faced personal tragedies, including the loss of his mother in 1925 and the death of his wife in 1952. These losses undoubtedly shaped his worldview and reinforced his commitment to his cause.
Borel was known for his charm and wit, often using humor to lighten difficult situations. Many remember him not only for his scientific mind but also for his warmth and accessibility. His ability to connect with people across different social and academic strata made him a respected and beloved figure in French intellectual circles.
Borel continued his active life well into his later years, maintaining a robust schedule of lectures, writing, and activism. His final years were spent working on his memoirs and continuing to champion the values of rational thinking and ethical conduct. On December 3, 1956, he passed away in Paris, leaving behind a legacy that extends far beyond his mathematical theorems and equations.
Émile Borel’s life and work exemplify the intertwining of intellectual endeavor and ethical responsibility. From his groundbreaking contributions to mathematics to his advocacy for rational thinking and social justice, Borel’s life story is one of constant pursuit of truth and the application of rigorous logic to better understand and improve the human condition.
Borel’s Posthumous Recognition and Modern Impact
Émile Borel's legacy has endured well beyond his lifetime, continuing to inspire mathematicians, scientists, and thinkers around the world. His work remains relevant in contemporary mathematical and scientific communities, providing a foundation for cutting-edge research in probability, set theory, and theoretical physics.
In the realm of probability theory, Borel's concepts continue to influence developments in stochastic processes and risk analysis. His pioneering work on Borel sets remains a cornerstone of measure theory and is fundamental in advanced courses on probability and statistics. Researchers and practitioners in fields such as finance, engineering, and data science frequently draw upon Borel's contributions to model and analyze complex systems.
Borel’s ideas on the infinitude and randomness found in natural phenomena continue to resonate with scholars in various disciplines. His exploration of normal numbers and the concept of randomness have implications not only for mathematics but also for philosophy and cognitive science. The study of how randomly distributed features manifest in the physical world has sparked ongoing interest and research, particularly in the context of artificial intelligence, where understanding randomness is crucial for developing robust algorithms.
Émile Borel's advocacy for ethical thinking and rational decision-making in mathematics and science has left a lasting legacy. His writings on ethics and the moral responsibilities of scientists continue to be cited and debated. His views on the importance of logical reasoning in resolving ethical dilemmas are particularly pertinent in the era of rapid technological advancement, where ethical considerations in data science, AI, and biotechnology are increasingly important.
Borel’s Influence on Subsequent Generations
Borel's influence on subsequent generations is profound and multifaceted. His students, colleagues, and fellow mathematicians have carried forward his ideas, building upon his foundational work to explore new frontiers in mathematics. Renowned mathematicians such as András Kündi, who studied under Borel, have perpetuated his legacy by advancing the fields of measure theory and stochastic processes.
Moreover, Borel's emphasis on the practical applications of mathematics has inspired countless students to pursue careers in both academia and industry. His belief in the power of rigorous mathematical reasoning to address real-world problems continues to motivate young mathematicians and scientists. The legacy of Émile Borel can be seen in the numerous mathematical journals, conferences, and textbooks dedicated to honoring his contributions.
The Borel Library and Archives
To preserve the memory of Émile Borel and his work, the Émil Borel Institute, located in Paris, houses a comprehensive collection of his writings, manuscripts, and correspondence. The institute also conducts research and educational programs that highlight Borel's achievements and promote ongoing studies in areas such as probability and mathematical logic. The Borel Library serves as a repository for scholars and enthusiasts alike, ensuring that Borel's legacy continues to inspire new generations of mathematicians and philosophers.
Conclusion: The Enduring Legacy of Émile Borel
In summary, Émile Borel was a multifaceted individual who left an indelible mark on the scientific and intellectual landscape of the 20th century. His contributions to mathematics, probability, and ethical thinking continue to shape contemporary discourse in various scientific and philosophical domains. Through his rigorous application of logical reasoning and ethical reflection, Borel demonstrated the power of mathematics to not only solve complex problems but also contribute to the betterment of society.
Émile Borel's legacy stands as a testament to the potential of interdisciplinary inquiry and the importance of applying mathematical principles to real-world challenges. As we celebrate his achievements, we also recognize the continuing relevance of his ideas in our rapidly evolving world. Borel's enduring impact serves as a reminder that the pursuit of truth and the application of rationality remain vital in addressing the complexities and uncertainties of the modern era.
Glenn Seaborg: The Life and Legacy of a Nobel Scientist
Introduction to a Scientific Icon
Glenn T. Seaborg stands as one of the most influential scientists of the 20th century. His groundbreaking work in nuclear chemistry reshaped modern science, earning him a Nobel Prize and a lasting legacy. This article explores his life, contributions, and the enduring impact of his discoveries.
Early Life and Education
Born in 1912 in Ishpeming, Michigan, Seaborg exhibited an early passion for science. He pursued chemistry at UCLA, where his brilliance quickly became evident. His academic journey laid the foundation for his future achievements in nuclear research.
Key Milestones in Seaborg’s Education
- Graduated from UCLA with a degree in chemistry
- Earned his Ph.D. from the University of California, Berkeley
- Began his lifelong association with the Lawrence Berkeley National Laboratory
The Discovery of Plutonium
Seaborg’s most famous achievement came in 1940, when he and his team discovered plutonium. This element became crucial in the development of nuclear energy and weapons, marking a turning point in scientific history.
Impact of Plutonium on Modern Science
The discovery of plutonium had far-reaching consequences:
- Enabled the creation of the atomic bomb, altering global power dynamics
- Paved the way for nuclear energy, a key component of modern power generation
- Established Seaborg as a leading figure in nuclear chemistry
Nobel Prize and Later Contributions
In 1951, Seaborg was awarded the Nobel Prize in Chemistry for his work on transuranium elements. His research didn’t stop there—he continued to expand the periodic table, discovering several new elements.
Seaborg’s Elements on the Periodic Table
His contributions include the discovery or co-discovery of:
- Plutonium (Pu)
- Americium (Am)
- Curium (Cm)
- Berkelium (Bk)
- Californium (Cf)
Legacy and Influence
Seaborg’s work extended beyond the lab. He served as chairman of the U.S. Atomic Energy Commission and advised multiple presidents. His dedication to science education inspired generations of researchers.
Honors and Recognitions
Among his many accolades:
- Element 106 was named Seaborgium (Sg) in his honor
- Received the National Medal of Science
- Inducted into the National Inventors Hall of Fame
Conclusion: A Lasting Scientific Legacy
Glenn Seaborg’s contributions to science remain unparalleled. From the discovery of plutonium to his role in shaping nuclear policy, his work continues to influence technology, energy, and global security. His story is a testament to the power of curiosity and innovation.
Stay tuned for Part 2, where we delve deeper into Seaborg’s political influence and his impact on nuclear energy development.
Seaborg’s Role in Nuclear Policy and Global Security
Beyond his scientific breakthroughs, Glenn Seaborg played a pivotal role in shaping U.S. nuclear policy. As chairman of the Atomic Energy Commission (AEC) from 1961 to 1971, he influenced key decisions during the Cold War era. His leadership helped establish frameworks for nuclear safety, arms control, and the peaceful use of atomic energy.
Key Policy Contributions
- Advised Presidents Kennedy, Johnson, and Nixon on nuclear strategy
- Championed the Non-Proliferation Treaty (NPT) to limit nuclear weapons spread
- Promoted civilian nuclear energy programs, including the development of nuclear power plants
The Science Behind Seaborg’s Discoveries
Seaborg’s work revolutionized our understanding of the periodic table. His research focused on transuranium elements—elements heavier than uranium—expanding the boundaries of chemistry. By bombarding uranium with neutrons, he and his team synthesized new elements, proving their existence through meticulous experimentation.
Breakthrough Techniques
Seaborg employed innovative methods, including:
- Neutron capture to create heavier isotopes
- Chemical separation to isolate new elements
- Radiation detection to confirm elemental properties
Seaborg’s Impact on Modern Energy
The discovery of plutonium was a game-changer for energy production. Today, nuclear power generates about 10% of the world’s electricity, with plutonium playing a critical role in reactor fuel. Seaborg’s work laid the foundation for sustainable energy solutions, reducing reliance on fossil fuels.
Nuclear Energy by the Numbers
- 440+ nuclear reactors operate globally, powering millions of homes
- Nuclear energy produces zero carbon emissions, aiding climate goals
- The U.S. alone generates over 800 billion kWh annually from nuclear power
Educational Legacy and Mentorship
Seaborg was deeply committed to science education. He mentored countless students at UC Berkeley, many of whom became leading scientists. His teaching philosophy emphasized hands-on research and interdisciplinary collaboration, shaping future generations of chemists and physicists.
Notable Students and Protégés
- Darryle J. Busch, a renowned inorganic chemist
- Albert Ghiorso, co-discoverer of multiple elements
- Edwin McMillan, Nobel laureate and colleague in transuranium research
Challenges and Controversies
Despite his achievements, Seaborg faced criticism for his role in nuclear weapons development. The atomic bomb raised ethical debates about scientific responsibility. Seaborg defended his work, arguing that nuclear deterrence was necessary for global stability, but he also advocated for arms control.
Public Perception and Debates
- Some viewed his contributions as essential for national security
- Critics questioned the moral implications of nuclear weapons
- Seaborg later supported disarmament efforts to reduce nuclear threats
Seaborg’s Later Years and Final Contributions
Even after retiring from the AEC, Seaborg remained active in science. He continued research at UC Berkeley, published over 500 scientific papers, and advocated for federal funding in education. His later work focused on nuclear medicine, exploring radioactive isotopes for cancer treatment.
Lasting Achievements
- Co-authored the “Seaborg Report” on nuclear waste management
- Received the Enrico Fermi Award for lifetime contributions
- Remained a public advocate for science until his passing in 1999
In Part 3, we’ll explore Seaborg’s cultural impact, including his appearances in media, his influence on pop culture, and the enduring relevance of his discoveries in today’s scientific landscape.
Seaborg’s Cultural and Media Influence
Glenn Seaborg wasn’t just a scientist—he became a cultural icon. His work appeared in documentaries, textbooks, and even popular media, shaping public perception of nuclear science. His discoveries were featured in films like “The Day After Trinity”, highlighting the dual nature of nuclear power: its potential for destruction and progress.
Seaborg in Books and Documentaries
- Featured in “The Making of the Atomic Bomb” by Richard Rhodes
- Subject of the PBS documentary “Modern Marvels: The Atom”
- Mentioned in “The Disappearing Spoon” by Sam Kean, a book on the periodic table
The Seaborg Effect: Inspiring Future Scientists
Seaborg’s legacy extends beyond his discoveries—he inspired generations of scientists. His story is taught in schools worldwide, demonstrating how curiosity and perseverance can change the world. Many young researchers cite him as a role model, particularly in STEM education.
Programs Named in His Honor
- The Glenn T. Seaborg Center in Michigan promotes science literacy
- The Seaborg Institute at Lawrence Livermore National Laboratory
- Numerous scholarships and awards for chemistry students
Seaborg’s Role in Nuclear Medicine
Beyond energy and weapons, Seaborg’s research contributed to medical advancements. His work on radioactive isotopes led to breakthroughs in cancer treatment and diagnostic imaging. Today, isotopes like plutonium-238 are used in pacemakers and radiation therapy.
Medical Applications of His Discoveries
- Radiation therapy for cancer patients
- Nuclear imaging techniques like PET scans
- Isotope-powered medical devices
Debates and Ethical Considerations
Seaborg’s work sparked ongoing debates about scientific ethics. While his discoveries advanced technology, they also raised questions about responsibility. Should scientists be held accountable for how their inventions are used? Seaborg himself grappled with this, advocating for peaceful applications of nuclear science.
Key Ethical Questions
- Balancing national security with global safety
- The moral implications of nuclear weapons
- Ensuring responsible innovation in science
The Future of Seaborg’s Legacy
Today, Seaborg’s influence persists in modern science. Researchers continue to explore transuranium elements, and nuclear energy remains a critical topic in climate discussions. His life reminds us that science is not just about discovery—it’s about impact, responsibility, and legacy.
Current Research Inspired by Seaborg
- New element synthesis at laboratories like CERN
- Advancements in nuclear fusion technology
- Innovations in radioactive waste management
Conclusion: A Life of Discovery and Influence
Glenn Seaborg’s journey—from a small-town student to a Nobel Prize-winning scientist—is a testament to the power of human ingenuity. His discoveries reshaped energy, medicine, and global policy, leaving an indelible mark on history. As we face modern challenges like climate change and energy security, his work remains more relevant than ever.
Seaborg once said,
“The most exciting phrase to hear in science, the one that heralds new discoveries, is not ‘Eureka!’ but ‘That’s funny…’”His legacy encourages us to keep asking questions, pushing boundaries, and striving for a better future through science.
From the discovery of plutonium to his role in nuclear policy, Glenn Seaborg’s story is one of curiosity, innovation, and enduring impact—a true icon of 20th-century science.