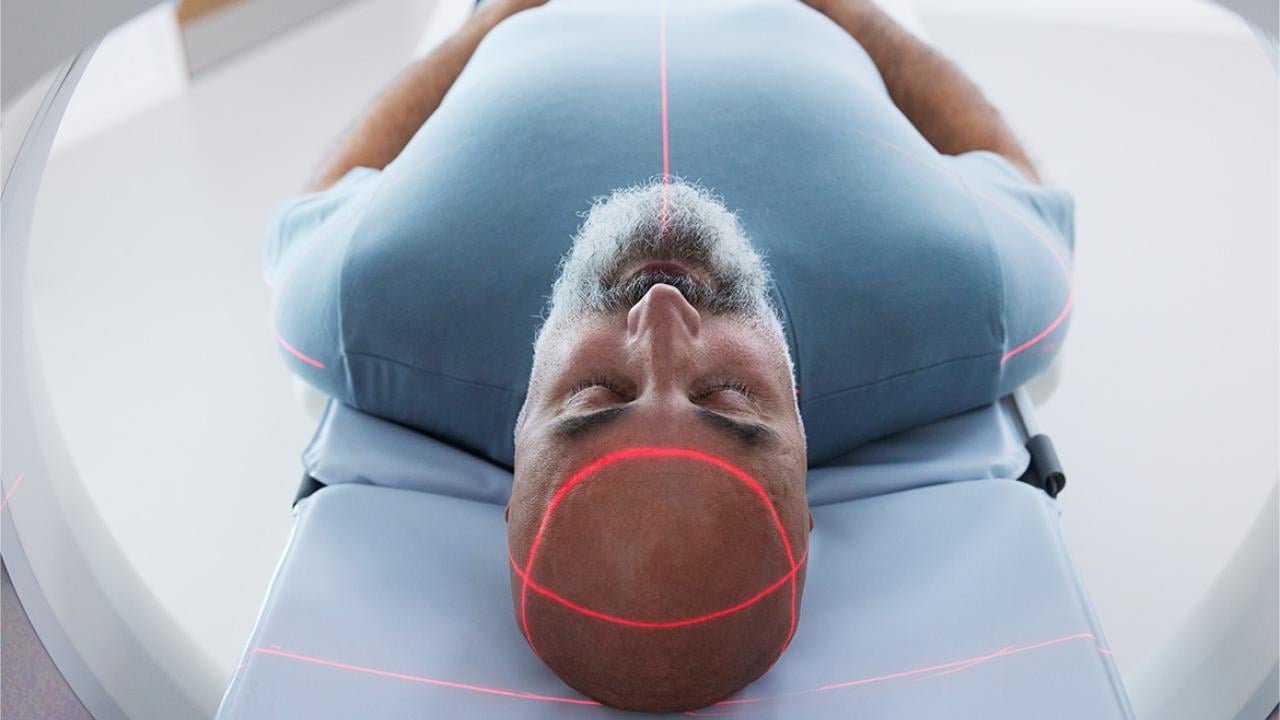
AI in Medical Physics: The Quiet Revolution in Healthcare
The scan revealed a tumor, a faint gray smudge nestled against the brainstem. For a human planner, mapping a precise radiation beam to destroy it while sparing the critical nerves millimeters away would consume hours of meticulous, painstaking work. On a screen at Stanford University in July 2024, an artificial intelligence finished the task in under a minute. The plan it generated wasn't just fast; it was clinically excellent, earning a "Best in Physics" designation from the American Association of Physicists in Medicine. This isn't a glimpse of a distant future. It is the documented present. A profound and quiet revolution is unfolding in the basements and control rooms of hospitals worldwide, where medical physics meets artificial intelligence.
The Invisible Architect of Precision
Medical physics has always been healthcare's silent backbone. These specialists ensure the massive linear accelerators that deliver radiation therapy fire with sub-millimeter accuracy. They develop the algorithms that transform raw MRI signals into vivid anatomical maps. Their work is the bridge between abstract physics and human biology. For decades, progress was incremental—faster processors, sharper detectors. Then machine learning arrived, not as a replacement, but as a force multiplier. AI is becoming the invisible architect of precision, redesigning workflows that have stood for thirty years.
The change is most visceral in radiation oncology. Traditionally, treatment planning is a brutal slog. A medical physicist or dosimetrist must manually "contour" or draw the borders of a tumor and two dozen sensitive organs-at-risk on dozens of CT scan slices. Then begins the iterative dance of configuring radiation beams—their angles, shapes, and intensities—to pour a lethal dose into the tumor while minimizing exposure to everything else. A single plan can take a full day.
“Our foundation model for radiotherapy planning represents a paradigm shift, not just an incremental improvement,” says Dr. Lei Xing, a professor of radiation oncology and medical physics at Stanford. “It learns from the collective wisdom embedded in tens of thousands of prior high-quality plans. The system doesn't just automate drawing; it understands the clinical goals and trade-offs, generating a viable starting point in seconds, not hours.”
This is the crux. The AI, particularly the foundation model highlighted at the 2024 AAPM meeting, isn't following a simple flowchart. It has ingested a vast library of human expertise. It recognizes that a prostate tumor plan prioritizes sparing the rectum and bladder, while a head-and-neck case involves a labyrinth of salivary glands and spinal cord. The output is a first draft, but one crafted by a peerless, instantaneous resident who has seen every possible variation of the disease. The human expert is elevated from drafter to editor, focusing on nuance and exception.
From Pixels to Prognosis: AI's Diagnostic Gaze
While therapy planning is one frontier, diagnostic imaging is another. The FDA has now cleared nearly 1,000 AI-enabled devices for radiology. Their function ranges from the administrative—prioritizing critical cases in a worklist—to the superhuman. One cleared algorithm can detect subtle bone fractures on X-rays that the human eye, weary from a hundred normal scans, might miss. Another performs a haunting task: reviewing past brain MRIs of epilepsy patients to find lesions that were originally overlooked. A 2024 study found such a tool successfully identified 64% of these missed lesions, potentially offering patients a long-delayed structural explanation for their seizures and a new path to treatment.
This capability moves medicine from reactive to proactive. It transforms the image from a static picture into a dynamic data mine. AI can quantify tumor texture, measure blood flow patterns in perfusion scans, or track microscopic changes in tissue density over time—variables too subtle or numerous for even the most trained specialist to consistently quantify. The pixel becomes a prognosis.
“The narrative is evolving from ‘AI versus radiologist’ to ‘AI augmenting the medical physicist and physician,’” notes a technical lead from the International Atomic Energy Agency (IAEA), which launched a major global webinar series on AI in radiation medicine in early 2024, drawing over 3,200 registrants. “Our focus is on educating medical physicists to become the essential human-in-the-loop, the validators and integrators who understand both the clinical question and the algorithm's limitations.”
This educational push is critical. The algorithms are tools, but profoundly strange ones. They don't reason like humans. A neural network might fixate on an irrelevant watermark on a scan template if it correlates with a disease in its training data, leading to bizarre and dangerous errors. The medical physicist’s new role is part engineer, part translator, and part quality assurance officer, ensuring these powerful but opaque systems are aligned with real-world biology.
The Engine of Innovation: 2024's Inflection Point
Something crystallized in 2024. The conversation moved from speculative journals to installed clinical software. The Stanford foundation model is a prime example. So is the rapid adoption of AI for real-time "adaptive radiotherapy" on MR-Linac machines. These hybrid devices combine an MRI scanner with a radiation machine, allowing clinicians to see a tumor's position in real-time immediately before treatment. But a problem remained: you could see the tumor move, but could you replan the radiation fast enough to hit it?
AI provides the answer. New systems can take the live MRI, automatically re-contour the shifted tumor and organs, and generate a completely new, optimized radiation plan in under five minutes. The therapy adapts to the patient’s anatomy on that specific day, accounting for bladder filling, bowel movement, or tumor shrinkage. This is a leap from static, pre-planned medicine to dynamic, responsive treatment. Research presented in 2023 even showed the potential for AI to analyze advanced diffusion-weighted MRI sequences on the Linac to identify and target the most radiation-resistant sub-regions within a glioblastoma, a notoriously aggressive brain tumor.
Meanwhile, in nuclear medicine, AI is enabling techniques once considered fantasy. "Multiplexed PET" imaging, a novel concept accelerated by AI algorithms, allows for the simultaneous use of multiple radioactive tracers in a single scan. Imagine injecting tracers for tumor metabolism, hypoxia, and proliferation all at once. Historically, their signals would blur together. AI, trained to recognize each tracer's unique temporal and spectral signature, can untangle them. This provides a multifaceted biological portrait of a tumor from one imaging session, without a single hardware change to the multi-million-dollar scanner. It’s a software upgrade that fundamentally alters diagnostic capability.
The pace is disorienting. One month, an AI is designing new drug molecules for lung fibrosis (the first entered Phase II trials in 2023). The next, it's compressing a week's worth of radiation physics labor into a coffee break. For the medical physicists at the center of this storm, the challenge is no longer just understanding the physics of radiation or the principles of MRI. It is now about mastering the logic of data, the architecture of neural networks, and the ethics of automated care. The silent backbone of healthcare is now its most dynamic engine of innovation.
The Digital Twin and the Deepening Divide
If the first wave of AI in medicine was about automation—drawing contours faster, prioritizing scans—the second wave is about simulation. The frontier is no longer the algorithm that assists but the model that predicts. Enter the in silico twin, or IST. This isn't a simple avatar. It is a dynamic, data-driven computational model of an individual patient’s physiology, built from their medical images, genetic data, and real-time biosensor feeds. The concept vaults over the limitations of population-based medicine. We are no longer treating a lung cancer patient based on averages from a thousand-patient trial. We are treating a specific tumor in a specific lung, with its unique blood supply and motion pattern, simulated in a virtual space where mistakes carry no cost.
"For clinicians, ISTs provide a testbed to simulate drug responses, assess risks of complications, and personalize treatment strategies without exposing patients," explains a comprehensive 2024 review of AI-powered ISTs published by the National Institutes of Health. The potential is staggering.
Research from 2024 and 2025 details models already in development: a liver twin that simulates innervation and calcium signaling for precision medicine; a lung digital twin for thoracic health monitoring that can predict ventilator performance; cardiac twins that map heart dynamics for surgical planning. In radiation oncology, this is the logical extreme of adaptive therapy. An IST could ingest a patient's daily MRI from the treatment couch, run a thousand micro-simulations of different radiation beam configurations in seconds, and present the optimal plan for that specific moment, accounting for organ shift, tumor metabolism, and even predicted cellular repair rates.
A study in the Journal of Applied Clinical Medical Physics with the DOI 10.1002/acm2.70395 provides a concrete stepping stone. Researchers developed a deep-learning framework trained on images from 180 patients to predict the optimal adaptive radiotherapy strategy. Should the team do a full re-plan, a simple shift, or something in between? The AI makes that classification in seconds, a task that typically consumes manual deliberation. It covers 100% of strategy scenarios, acting as a tireless, instant second opinion for every single case.
The Cracks in the Foundation
This is where the hype meets a wall of sober, scientific skepticism. For every paper heralding a new digital twin, a murmur grows among fundamental scientists. The promise is a system that understands human biology. The reality, argue some, is a system that is profoundly good at pattern recognition but hopelessly ignorant of the laws of physics and biology that govern that pattern.
"Current architectures—large language models... fail to capture even elementary scientific laws," states a provisionally accepted 2025 paper in Frontiers in Physics by Peter V. Coveney and Roger Highfield. Their critique is damning and foundational. "The impact of AI on physics has been more promotional than technical."
This is the central, unresolved tension. An AI can be trained on a million MRI scans and learn to contour a liver with superhuman consistency. But does it understand why the liver has that shape? Does it comprehend the fluid dynamics of blood flow, the biomechanical properties of soft tissue, the principles of radiation transport at the cellular level? Almost certainly not. It has learned a statistical correlation, not a mechanistic truth. This matters immensely when that AI is asked to extrapolate—to predict how a never-before-seen tumor will respond to a novel radiation dose, or to simulate a drug's effect in a cirrhotic liver when it was only trained on healthy tissue. It will fail, often silently and confidently.
The medical physics community is thus split. One camp, the engineers, sees immense practical utility in tools that work reliably 95% of the time, freeing them for the 5% of edge cases. The other camp, the physicist-scientists, fears building a clinical edifice on a foundation of sophisticated correlation, mistaking it for causation. What happens when the algorithm makes a catastrophic error? No one can peer inside its "black box" to trace the flawed logic. You cannot debate with a neural network.
Integration and the Burden of Validation
Beyond the philosophical debate lies the gritty, operational challenge of integration. The International Atomic Energy Agency's six-month global webinar series, launched in 2024 and attracting over 3,200 registrants, wasn't about selling dreams. It was a direct response to a palpable skills gap. Hospitals are purchasing AI tools with seven-figure price tags, and clinical staff are expected to use them. But who validates the output? Who ensures the AI hasn't been poisoned by biased training data that underperforms on patients of a certain ethnicity or body habitus? The answer, increasingly, is the medical physicist.
Their job description is morphing. They are no longer just the custodians of the linear accelerator's beam calibration. They are becoming the required "human-in-the-loop" for a suite of autonomous systems. This requires a new literacy. They must understand enough about convolutional neural networks, training datasets, and loss functions to perform clinical validation. They must establish continuous quality assurance protocols for software that updates itself. A tool that worked perfectly in October might behave differently after a "minor improvement" pushed by the vendor in November. The physicist is the last line of defense.
"The IAEA initiative recognizes that the bottleneck is no longer AI development, but AI education," notes a coordinator of the series. "We are turning medical physicists into the essential bridge, the professionals who can translate algorithmic confidence into clinical certainty."
This validation burden slows adoption to a frustrating crawl for technologists. A tool can show spectacular results in a retrospective study, yet face months or years of prospective clinical validation before it is trusted with real patients. This is where pilot programs, like those for ICU-based digital twins for ventilator management or glucose control, are critical. They operate in controlled, monitored environments, generating the real-world evidence needed for broader rollout. Some ISTs are already finding a foothold in regulatory science, used in physiologically-based pharmacokinetic (PBPK) modeling to predict drug interactions—a quiet endorsement of their predictive power.
But the workflow change is cultural. Adopting an AI contouring tool means a radiation oncologist must relinquish the ritual of manually drawing the tumor target, a act that embodies their diagnostic authority. They must learn to edit, not create. This shift requires humility and trust, commodities in short supply in high-stakes medicine. The most successful integrations happen where the AI is framed not as an oracle, but as a super-powered resident—always fast, sometimes brilliant, but always requiring attending supervision.
A Question of Agency and the 2026 Horizon
Look ahead to 2026. The chatter at conferences like the New York Academy of Sciences' "The New Wave of AI in Healthcare 2026" event points to a new phase: agentic AI. These are not single-task models for contouring or dose prediction. They are orchestrators. Imagine a system that, upon a lung cancer diagnosis, automatically retrieves the patient's CT, PET, and genomic data, launches an IST simulation to model tumor growth under different regimens, drafts a fully optimized radiation therapy plan, schedules the treatments on the linear accelerator, and generates the clinical documentation. It manages the entire workflow, requesting human input only at defined decision points.
This is the promise of streamlined, error-free care. It is also the nightmare of deskilled clinicians and systemic opacity. If a treatment fails, who is responsible? The oncologist who approved the AI's plan? The physicist who validated the system? The software company? The legal framework is a quagmire.
"The growth is in applications that integrate multimodal data for real-time care coordination," predicts a 2026 trends report from Mass General Brigham, highlighting the move toward these agentic systems in imaging-heavy fields. The goal is a cohesive, intelligent healthcare system. The risk is a brittle, automated pipeline that amplifies hidden biases.
We stand at a peculiar moment. The tools are here. Their potential is undeniable. A patient today can receive a radiation plan shaped by an AI that has learned from the collective experience of every top cancer center in the world. Yet, the very scientists who understand the underlying physics warn us that these tools lack a fundamental understanding of the reality they manipulate. The medical physicist is now tasked with an impossible duality: be the enthusiastic adopter of transformative technology, and be its most rigorous, skeptical interrogator. They must harness the power of the correlation engine while remembering, every single day, that correlation is not causation. The future of precision medicine depends on whether they can hold both those truths in mind without letting either one go.
The Redefinition of Expertise and the Human Mandate
The significance of AI in medical physics transcends faster software or sharper images. It represents a fundamental renegotiation of the contract between human expertise and machine intelligence in one of society's most trusted domains. For a century, the authority of the clinic rested on the trained judgment of the specialist—the radiologist’s gaze, the surgeon’s hand, the physicist’s calculation. That authority is now distributed, parsed between the clinician and the algorithm. The cultural impact is a quiet but profound shift in how we define medical error, clinical responsibility, and even the nature of healing. Is a treatment plan "better" because it conforms to established human protocol, or because an inscrutable model predicts a 3% higher survival probability? The field is building the answer in real time, case by case.
This revolution is also industrial. It promises to alleviate crushing workforce shortages by elevating the role of every remaining expert. A single medical physicist, armed with validated AI tools, could oversee the technical quality of treatments across multiple clinics, bringing elite-level precision to underserved communities. The historical legacy here isn't just about curing more cancer. It's about democratizing the highest standard of care. The 2024 IAEA webinars, attracting thousands globally, weren't a technical seminar. They were an attempt to level the playing field, ensuring that a hospital in Jakarta or Nairobi has the same literacy in these tools as one in Boston.
"The transition we are managing is from the medical physicist as an operator of machines to the medical physicist as a conductor of intelligent systems," observes a lead physicist at a major European cancer center who has integrated multiple AI platforms. "Our value is no longer in turning the knobs ourselves, but in knowing which knobs the AI should turn, and when to slap its hand away from the console."
This redefinition strikes at the core of professional identity. The pride of craft in meticulously crafting a radiation plan is being replaced by the pride of judgment in validating one. It's a less visceral, more intellectual satisfaction, and the transition is generating a quiet unease. The field is grappling with a paradox: to stay relevant, its practitioners must cede the very tasks that once defined their relevance.
The Uncomfortable Truths and Unanswered Questions
For all the promise, the critical perspective cannot be glossed over. The "black box" problem isn't a technical hiccup; it's a philosophical deal-breaker for a science built on reproducibility and mechanistic understanding. We are implementing systems whose decision-making process we cannot fully audit. When a deep learning model for adaptive therapy selects a novel beam arrangement, can we trace that choice back to a physical principle about tissue absorption, or is it echoing a statistical ghost in its training data? The Coveney and Highfield critique in Frontiers in Physics lingers like a specter: these models lack the foundational physics they are meant to apply.
The economic model is another fissure. The proliferation of proprietary, "locked" AI tools risks creating a new kind of healthcare disparity—not just in access to care, but in access to understanding the care being delivered. A hospital may become dependent on a vendor's algorithm whose inner workings are a trade secret. How does a physicist perform independent quality assurance on a sealed unit? This commodification of core medical judgment could erode the profession's autonomy, turning clinicians into gatekeepers for corporate intellectual property.
Furthermore, the data hunger of these models creates perverse incentives. The most powerful AI will be built by the institutions with the largest, most diverse patient datasets. This risks cementing the dominance of already-privileged centers and baking their historical patient demographics—with all the biases that entails—into the global standard of care. An AI trained primarily on North American and European populations may falter when presented with anatomical or disease presentations more common in other parts of the world, a form of algorithmic colonialism delivered through a hospital's PACS system.
The Path Beyond the Hype Cycle
The Gartner Hype Cycle prediction that medical AI will slide into the "Trough of Disillusionment" by 2026 is not a doom forecast. It's a necessary correction. The next two years will separate theatrical demos from clinical workhorses. The focus will shift from publishing papers on model accuracy to publishing long-term outcomes on patient survival and quality of life. The conversation at the 2026 New York Academy of Sciences symposium and similar gatherings will be less about AI's potential and more about its proven, measurable impact on hospital readmission rates, treatment toxicity, and cost.
Concrete developments are already on the calendar. The next phase of the IAEA's initiative will move from webinars to hands-on, validated implementation frameworks. Regulatory bodies like the FDA are developing more nuanced pathways for continuous-learning AI, moving beyond the one-time clearance of a static device. And in research labs, the push for "explainable AI" (XAI) is gaining urgent momentum. The goal is not just a model that works, but one that can articulate, in terms a physicist can understand, the *why* behind its recommendation.
The most immediate prediction is the rise of the hybrid physicist-data scientist. Graduate programs in medical physics are already scrambling to integrate mandatory coursework in machine learning, statistics, and data ethics. The physicist of 2027 will be bilingual, fluent in the language of Monte Carlo simulations *and* convolutional neural networks. Their primary instrument will no longer be the ion chamber alone, but the integrated dashboard that displays both radiation dose distributions and the confidence intervals of the AI that helped generate them.
In a control room at Stanford or Mass General, the scene is already changing. The glow of the monitor illuminates not just a CT scan, but a parallel visualization: the patient’s anatomy, the AI-proposed dose cloud in vivid color, and a sidebar of metrics quantifying the algorithm's certainty. The physicist’s hand rests not on a mouse to draw, but on a trackpad to navigate layers of data. They are reading the story of a disease, written in biology but annotated by silicon. The machine offers a brilliant, lightning-fast draft. The human provides the wisdom, the caution, the context of a life. That partnership, fraught and imperfect, is the new engine of care. The question is no longer whether AI will change medical physics. The question is whether we can build a science—and an ethics—robust enough to handle the change.
Ernst Ruska: The Father of Electron Microscopy
Ernst Ruska, a pioneering German physicist, revolutionized the field of microscopy with his invention of the electron microscope. His groundbreaking work in the early 20th century laid the foundation for modern imaging technologies, enabling scientists to explore the microscopic world at unprecedented resolutions.
Early Life and Education
Born in 1906, Ernst Ruska showed an early aptitude for science and engineering. He pursued his studies at the Technical University of Munich and later at the Technische Hochschule Berlin, where he delved into high-voltage research and cathode-ray oscillograph calculations. His academic journey was marked by a keen interest in the behavior of electrons and their potential applications in imaging.
Academic Foundations
Ruska's early work was influenced by the theories of Hans Busch, who in 1926 proposed that magnetic fields could bundle electrons in a manner similar to how lenses focus light. This concept became a cornerstone of Ruska's later inventions. During his studies, he also collaborated with Max Knoll, a partnership that would prove instrumental in the development of the electron microscope.
The Invention of the Electron Microscope
The electron microscope was a monumental leap forward in imaging technology. Unlike traditional optical microscopes, which are limited by the wavelength of visible light, electron microscopes use beams of electrons to achieve far greater resolutions. This innovation allowed scientists to observe structures at the atomic level, opening new avenues in fields such as biology, materials science, and nanotechnology.
Key Milestones
On March 9, 1931, Ruska and Knoll achieved a significant breakthrough: the first two-stage electron-optical magnification using magnetic lenses. This milestone was built on Busch's earlier theories and marked the beginning of a new era in microscopy. By December 1933, Ruska's prototype had already surpassed the resolution capabilities of light microscopes, achieving a magnification of 12,000x.
- 1931: First two-stage electron-optical magnification
- 1933: Prototype exceeds light microscope resolution
- 1938–1939: First serial-production electron microscope developed at Siemens
Commercialization and Impact
With the assistance of Bodo von Borries, Ruska developed the first commercially viable electron microscope at Siemens. This instrument enabled atomic-scale imaging, revolutionizing scientific research and industrial applications. The ability to visualize structures at such minute scales had a profound impact on various disciplines, from biology to materials science.
Recognition and Legacy
Ernst Ruska's contributions to science were recognized with numerous accolades, culminating in the Nobel Prize in Physics in 1986. He shared this prestigious award with Gerd Binnig and Heinrich Rohrer for their work on scanning tunneling microscopy. Ruska's electron microscope, initially termed the "Übermikroskop," has left an indelible mark on the scientific community, spurring advancements in nanotechnology, virology, and beyond.
Preservation and Influence
The original electron microscope developed by Ruska is preserved at the Deutsches Museum in Munich, serving as a testament to his ingenuity. Modern electron microscopy continues to evolve, integrating high-performance computing and AI-enhanced image processing to achieve dynamic 3D reconstructions and sub-angstrom resolutions. Educational videos and resources from 2023 highlight the ongoing evolution of electron microscopy, from Ruska's early prototypes to advanced techniques like scanning electron microscopy (SEM) and transmission electron microscopy (TEM).
Technical Innovations and Advancements
The electron microscope operates on the principle of using electrons instead of light to illuminate specimens. This approach leverages the much shorter wavelength of electrons, approximately 100,000 times shorter than that of visible light, to achieve superior resolution. The electrons are focused using magnetic lenses, a concept derived from Busch's theories, and deflected by atoms within the specimen to create contrast.
Resolution and Magnification
The resolution capabilities of electron microscopes are truly remarkable. While traditional light microscopes are limited to resolutions of about 200 nanometers, electron microscopes can achieve resolutions as fine as 0.1 nanometers. This leap in resolution has enabled scientists to visualize structures at the atomic level, providing unprecedented insights into the fundamental building blocks of matter.
"The electron microscope has revolutionized our understanding of the microscopic world, enabling us to see what was previously invisible."
Early prototypes of the electron microscope achieved magnifications of up to 12,000x, a feat that was unthinkable with light microscopes. Modern electron microscopes can exceed magnifications of millions-fold, allowing for detailed observations of complex structures such as proteins, viruses, and nanomaterials.
Applications and Impact
The impact of the electron microscope extends across numerous scientific disciplines. In biology, it has enabled the visualization of cellular structures, viruses, and macromolecules, providing critical insights into biological processes. In materials science, electron microscopy has facilitated the study of crystalline structures, defects, and nanomaterials, driving advancements in technology and engineering.
- Biology: Visualization of cellular structures and macromolecules
- Materials Science: Study of crystalline structures and nanomaterials
- Nanotechnology: Exploration of atomic-scale structures and properties
The advent of techniques such as cryo-electron microscopy and aberration-corrected lenses has further expanded the capabilities of electron microscopy. These advancements have enabled the visualization of protein structures at sub-angstrom resolutions and the creation of dynamic 3D reconstructions, pushing the boundaries of scientific exploration.
Conclusion
Ernst Ruska's invention of the electron microscope has had a transformative impact on science and technology. His pioneering work has enabled researchers to explore the microscopic world at unprecedented levels of detail, driving advancements in fields ranging from biology to materials science. As electron microscopy continues to evolve, incorporating cutting-edge technologies such as AI and high-performance computing, Ruska's legacy remains a cornerstone of modern scientific discovery.
Ernst Ruska's Contributions to Modern Science
Ernst Ruska's groundbreaking work on the electron microscope not only revolutionized imaging technology but also had a profound impact on various scientific disciplines. His invention enabled researchers to explore the microscopic world with unprecedented clarity, leading to significant advancements in fields such as biology, materials science, and nanotechnology.
Advancements in Biology
The electron microscope has been instrumental in the field of biology, allowing scientists to visualize cellular structures, viruses, and macromolecules at the atomic level. This capability has provided critical insights into biological processes, enabling researchers to better understand the fundamental mechanisms of life.
- Cellular Structures: Detailed imaging of organelles and intracellular components
- Virology: Visualization of viral particles and their interactions with host cells
- Macromolecules: Study of complex biological molecules such as proteins and nucleic acids
One of the most significant contributions of electron microscopy to biology has been in the field of virology. The ability to visualize viral particles has been crucial in understanding viral structures, replication mechanisms, and interactions with host cells. This knowledge has been instrumental in the development of vaccines and antiviral therapies.
Impact on Materials Science
In the realm of materials science, the electron microscope has enabled researchers to study the properties and behaviors of materials at the atomic scale. This has led to the development of new materials with enhanced properties, as well as a deeper understanding of the fundamental principles governing material behavior.
- Crystalline Structures: Analysis of atomic arrangements and defects in crystals
- Nanomaterials: Exploration of the unique properties of materials at the nanoscale
- Material Properties: Investigation of mechanical, electrical, and thermal properties
The electron microscope has been particularly valuable in the study of nanomaterials. The ability to visualize and manipulate materials at the nanoscale has led to the development of novel materials with unique properties, such as enhanced strength, conductivity, and reactivity. These advancements have had a significant impact on industries ranging from electronics to medicine.
The Evolution of Electron Microscopy
Since the invention of the first electron microscope by Ernst Ruska and Max Knoll in 1931, the technology has undergone significant advancements. Modern electron microscopes incorporate cutting-edge technologies such as high-performance computing, AI-enhanced image processing, and advanced imaging techniques, enabling researchers to explore the microscopic world with unprecedented detail and precision.
From Static to Dynamic Imaging
Early electron microscopes were limited to static imaging, providing two-dimensional snapshots of specimens. However, modern electron microscopy has evolved to include dynamic imaging capabilities, allowing researchers to observe processes and interactions in real-time. This has been particularly valuable in the study of biological systems, where dynamic processes such as cellular interactions and molecular dynamics can be visualized.
- 3D Imaging: Reconstruction of three-dimensional structures from two-dimensional images
- Time-Resolved Imaging: Observation of processes and interactions in real-time
- Correlative Microscopy: Integration of multiple imaging techniques for comprehensive analysis
One of the most significant advancements in electron microscopy has been the development of 3D imaging techniques. By combining multiple two-dimensional images, researchers can reconstruct three-dimensional structures, providing a more comprehensive understanding of complex systems. This capability has been particularly valuable in the study of biological macromolecules and cellular structures.
Integration of High-Performance Computing
The integration of high-performance computing has been a game-changer in the field of electron microscopy. Advanced computational techniques enable researchers to process and analyze large datasets, extract meaningful information, and create detailed reconstructions of complex structures. This has led to significant advancements in fields such as structural biology, where the visualization of protein structures at atomic resolutions has been made possible.
- Image Processing: Enhancement and analysis of electron microscope images
- Data Analysis: Extraction of meaningful information from large datasets
- Simulation and Modeling: Prediction and visualization of complex systems
The use of AI-enhanced image processing has further expanded the capabilities of electron microscopy. Machine learning algorithms can automatically identify and classify features within images, enabling researchers to analyze large datasets with greater efficiency and accuracy. This has been particularly valuable in the study of complex biological systems, where the identification of specific structures and interactions can be challenging.
Ernst Ruska's Legacy and Influence
Ernst Ruska's invention of the electron microscope has had a lasting impact on the scientific community, spurring advancements in numerous fields and inspiring generations of researchers. His pioneering work has been recognized with numerous accolades, including the Nobel Prize in Physics in 1986, and his legacy continues to shape the future of scientific discovery.
Recognition and Awards
Throughout his career, Ernst Ruska received numerous awards and honors in recognition of his contributions to science. In addition to the Nobel Prize, he was awarded the Lasker Award in 1960 and the Paul Ehrlich and Ludwig Darmstaedter Prize in 1970. These accolades reflect the profound impact of his work on the scientific community and the broader world.
- Nobel Prize in Physics (1986)
- Lasker Award (1960)
- Paul Ehrlich and Ludwig Darmstaedter Prize (1970)
The Nobel Prize in Physics awarded to Ruska in 1986 was a testament to the transformative impact of his invention. The prize was shared with Gerd Binnig and Heinrich Rohrer for their work on scanning tunneling microscopy, highlighting the broader significance of advancements in imaging technology.
Influence on Future Generations
Ruska's work has inspired generations of scientists and engineers, encouraging them to push the boundaries of scientific discovery. His invention of the electron microscope has not only revolutionized imaging technology but also opened new avenues for exploration and innovation. Today, electron microscopy continues to evolve, incorporating cutting-edge technologies and driving advancements in fields ranging from biology to materials science.
- Education: Inspiring students and researchers to pursue careers in science and engineering
- Innovation: Encouraging the development of new technologies and techniques
- Collaboration: Fostering interdisciplinary research and cooperation
The influence of Ernst Ruska extends beyond his technical achievements. His commitment to scientific exploration and innovation has served as a model for future generations, encouraging them to pursue their own groundbreaking discoveries. The electron microscope, once a revolutionary invention, has become an indispensable tool in modern science, and its continued evolution is a testament to Ruska's enduring legacy.
The Future of Electron Microscopy
The field of electron microscopy continues to evolve, driven by advancements in technology and the ongoing pursuit of scientific discovery. Modern electron microscopes incorporate cutting-edge techniques such as cryo-electron microscopy, aberration-corrected lenses, and AI-enhanced image processing, enabling researchers to explore the microscopic world with unprecedented detail and precision.
Emerging Technologies
One of the most promising developments in electron microscopy is the advent of cryo-electron microscopy. This technique involves flash-freezing specimens to preserve their natural structures, allowing researchers to visualize biological macromolecules in their native states. This capability has been particularly valuable in the study of protein structures, enabling researchers to achieve sub-angstrom resolutions and gain insights into the fundamental mechanisms of biological processes.
- Cryo-Electron Microscopy: Visualization of biological macromolecules in their native states
- Aberration-Corrected Lenses: Enhancement of resolution and image quality
- AI-Enhanced Image Processing: Automatic identification and classification of features
The development of aberration-corrected lenses has also been a significant advancement in electron microscopy. These lenses correct for optical aberrations, enhancing the resolution and image quality of electron microscopes. This has enabled researchers to achieve unprecedented levels of detail, providing new insights into the structures and behaviors of materials at the atomic scale.
Applications in Nanotechnology
The field of nanotechnology has benefited greatly from the advancements in electron microscopy. The ability to visualize and manipulate materials at the nanoscale has led to the development of novel materials with unique properties, as well as a deeper understanding of the fundamental principles governing nanoscale phenomena. This has had a significant impact on industries ranging from electronics to medicine, driving innovations in areas such as nanomedicine, nanoelectronics, and nanomaterials.
- Nanomedicine: Development of targeted drug delivery systems and diagnostic tools
- Nanoelectronics: Creation of advanced electronic devices and components
- Nanomaterials: Exploration of materials with unique properties at the nanoscale
The future of electron microscopy holds great promise, with ongoing advancements in technology and technique driving new discoveries and innovations. As researchers continue to push the boundaries of what is possible, the legacy of Ernst Ruska and his groundbreaking invention will continue to inspire and shape the future of scientific exploration.
The Enduring Impact of Ernst Ruska's Electron Microscope
The electron microscope invented by Ernst Ruska has fundamentally transformed scientific research, enabling breakthroughs that were once unimaginable. From its humble beginnings in the 1930s to its modern iterations, this technology continues to push the boundaries of human knowledge, allowing scientists to explore the atomic and molecular worlds with remarkable precision.
Revolutionizing Scientific Research
The impact of the electron microscope on scientific research cannot be overstated. Before its invention, scientists were limited by the resolution of optical microscopes, which could only magnify objects up to about 2000x. Ruska's electron microscope shattered this barrier, achieving magnifications of 12,000x by 1933 and eventually reaching millions-fold magnification in modern systems. This leap in capability has unlocked new frontiers in fields such as biology, chemistry, and materials science.
- Biology: Enabled the visualization of viruses, cellular structures, and macromolecules
- Chemistry: Facilitated the study of molecular structures and chemical reactions at the atomic level
- Materials Science: Allowed for the analysis of crystalline structures, defects, and nanomaterials
One of the most significant contributions of the electron microscope has been in the field of virology. For the first time, scientists could visualize viral particles in intricate detail, leading to a deeper understanding of viral structures and their interactions with host cells. This knowledge has been crucial in the development of vaccines and antiviral therapies, ultimately saving countless lives.
Advancements in Medical Science
The electron microscope has played a pivotal role in advancing medical science. By enabling the visualization of cellular and sub-cellular structures, it has provided invaluable insights into the mechanisms of diseases and the development of targeted therapies. For example, the study of protein structures using electron microscopy has led to breakthroughs in understanding diseases such as Alzheimer's and Parkinson's.
- Disease Research: Visualization of pathogens and disease mechanisms
- Drug Development: Design of targeted therapies based on molecular structures
- Diagnostic Tools: Development of advanced imaging techniques for medical diagnostics
The advent of cryo-electron microscopy has further revolutionized medical research. This technique allows scientists to visualize biological macromolecules in their native states, providing unprecedented insights into their structures and functions. This capability has been instrumental in the development of new drugs and therapies, as well as in the understanding of complex biological processes.
Ernst Ruska's Influence on Modern Technology
Ernst Ruska's invention of the electron microscope has not only advanced scientific research but also had a profound impact on modern technology. The principles and techniques developed for electron microscopy have been applied to a wide range of technologies, from semiconductor manufacturing to nanotechnology. This section explores the various ways in which Ruska's work has shaped the technological landscape.
Semiconductor Industry
The semiconductor industry has greatly benefited from the advancements in electron microscopy. The ability to visualize and manipulate materials at the atomic scale has been crucial in the development of integrated circuits and other electronic components. Electron microscopy has enabled engineers to analyze the structure and properties of semiconductor materials, leading to the creation of more efficient and powerful electronic devices.
- Integrated Circuits: Analysis and optimization of semiconductor structures
- Material Characterization: Study of material properties and defects
- Quality Control: Inspection and testing of electronic components
The use of scanning electron microscopy (SEM) and transmission electron microscopy (TEM) has become standard practice in the semiconductor industry. These techniques allow for the detailed analysis of semiconductor materials, enabling engineers to identify and correct defects, optimize performance, and develop new technologies.
Nanotechnology
The field of nanotechnology has been particularly transformed by the advancements in electron microscopy. The ability to visualize and manipulate materials at the nanoscale has led to the development of novel materials with unique properties, as well as the creation of advanced nanodevices. Electron microscopy has been instrumental in the study of nanomaterials, enabling researchers to explore their structures, properties, and behaviors.
- Nanomaterials: Exploration of materials with unique properties at the nanoscale
- Nanodevices: Development of advanced devices and components
- Nanoelectronics: Creation of electronic devices at the nanoscale
The development of aberration-corrected lenses has further enhanced the capabilities of electron microscopy in nanotechnology. These lenses correct for optical aberrations, enabling researchers to achieve unprecedented levels of detail and precision. This has led to significant advancements in the study of nanomaterials and the development of nanodevices, driving innovations in fields such as nanoelectronics and nanomedicine.
Preserving Ernst Ruska's Legacy
The legacy of Ernst Ruska and his groundbreaking invention continues to inspire and shape the future of scientific discovery. His work has been preserved and celebrated in various ways, ensuring that his contributions to science and technology are remembered and appreciated by future generations.
Museums and Exhibitions
The original electron microscope developed by Ruska is preserved at the Deutsches Museum in Munich, serving as a testament to his ingenuity and innovation. This historic artifact is a reminder of the transformative impact of Ruska's work and the enduring legacy of his invention. Museums and exhibitions around the world continue to showcase the evolution of electron microscopy, highlighting its significance in the history of science and technology.
- Deutsches Museum: Preservation of the original electron microscope
- Science Museums: Exhibitions on the history and evolution of electron microscopy
- Educational Programs: Initiatives to inspire future generations of scientists and engineers
Educational programs and initiatives have been developed to inspire future generations of scientists and engineers. These programs aim to foster a deeper understanding of the principles and applications of electron microscopy, encouraging students to pursue careers in science and technology. By preserving and promoting Ruska's legacy, these initiatives ensure that his contributions continue to inspire and shape the future of scientific discovery.
Educational Resources and Outreach
The importance of educational resources and outreach in preserving Ernst Ruska's legacy cannot be overstated. Educational videos, online courses, and interactive exhibits have been developed to provide students and researchers with a comprehensive understanding of electron microscopy and its applications. These resources aim to inspire and engage the next generation of scientists, ensuring that Ruska's work continues to have a lasting impact.
- Online Courses: Comprehensive courses on electron microscopy and its applications
- Interactive Exhibits: Hands-on experiences to explore the principles of electron microscopy
- Educational Videos: Engaging content to inspire and educate students and researchers
One notable example of educational outreach is the series of videos produced in 2023, which highlight the evolution of electron microscopy from Ruska's early prototypes to advanced techniques such as scanning electron microscopy (SEM) and transmission electron microscopy (TEM). These videos provide a compelling overview of the history and significance of electron microscopy, inspiring students and researchers to explore the microscopic world.
Conclusion: The Lasting Legacy of Ernst Ruska
Ernst Ruska's invention of the electron microscope has had a profound and lasting impact on the scientific community. His pioneering work has enabled researchers to explore the microscopic world with unprecedented detail and precision, driving advancements in fields ranging from biology to materials science. The electron microscope has become an indispensable tool in modern science, and its continued evolution is a testament to Ruska's enduring legacy.
Key Takeaways
The key takeaways from Ernst Ruska's contributions to science and technology are as follows:
- Revolutionary Invention: The electron microscope shattered the resolution barriers of optical microscopes, achieving magnifications of millions-fold.
- Transformative Impact: Enabled breakthroughs in biology, chemistry, materials science, and medical research.
- Technological Advancements: Drove innovations in semiconductor manufacturing, nanotechnology, and advanced imaging techniques.
- Inspiration for Future Generations: Ruska's work continues to inspire and shape the future of scientific discovery.
The electron microscope has not only revolutionized scientific research but also had a profound impact on modern technology. From the development of advanced electronic devices to the exploration of nanomaterials, Ruska's invention has driven innovations that have transformed industries and improved lives. His legacy serves as a reminder of the power of scientific curiosity and the potential for groundbreaking discoveries to shape the future.
The Future of Electron Microscopy
The future of electron microscopy holds great promise, with ongoing advancements in technology and technique driving new discoveries and innovations. Modern electron microscopes incorporate cutting-edge technologies such as high-performance computing, AI-enhanced image processing, and advanced imaging techniques, enabling researchers to explore the microscopic world with unprecedented detail and precision.
- Cryo-Electron Microscopy: Visualization of biological macromolecules in their native states.
- Aberration-Corrected Lenses: Enhancement of resolution and image quality.
- AI-Enhanced Image Processing: Automatic identification and classification of features.
As researchers continue to push the boundaries of what is possible, the legacy of Ernst Ruska and his groundbreaking invention will continue to inspire and shape the future of scientific exploration. The electron microscope, once a revolutionary invention, has become an indispensable tool in modern science, and its continued evolution is a testament to Ruska's enduring impact on the world of science and technology.
In conclusion, Ernst Ruska's contributions to science and technology have left an indelible mark on the world. His invention of the electron microscope has revolutionized scientific research, driven technological advancements, and inspired generations of scientists and engineers. As we look to the future, the continued evolution of electron microscopy serves as a reminder of the power of innovation and the potential for groundbreaking discoveries to transform our understanding of the world.