Dmitri Mendeleev: The Architect of the Periodic Table
Introduction to a Scientific Visionary
Dmitri Ivanovich Mendeleev, a name synonymous with the chemical marvel that is the periodic table, was more than just a scientist—he was a visionary who navigated the largely unexplored territories of chemistry in the late 19th century. Born on February 8, 1834, in Tobolsk, Siberia, Mendeleev's life journey was one of resilience, intelligence, and unyielding curiosity. His monumental contribution to chemistry revolutionized how scientists approached and understood elements, providing a systematic framework that continues to be integral to the field today.
Early Life and Education
Mendeleev was born into a large family, the youngest of 17 children. Following his father's early death, the family faced financial difficulties. His mother, Maria Dmitrievna Mendeleeva, was determined to provide him with educational opportunities, recognizing his potential. This unwavering support led the young Mendeleev to St. Petersburg, where he studied at the Main Pedagogical Institute.
His early education was marked by both hardship and brilliance. Despite suffering from tuberculosis, which led him to spend a significant amount of time in the milder climate of the Crimean Peninsula, Mendeleev graduated and soon after began a tenured position teaching chemistry. His keen mind and dedication to the discipline allowed him to soon dive deep into chemical research.
Mendeleev's Path to the Periodic Table
During the mid-19th century, the field of chemistry was rapidly expanding, with new elements being discovered and analyzed. Yet, there was no coherent system to organize them. Mendeleev's quest for order amid this increasing complexity would become his lifelong pursuit. He aimed to find a pattern that could connect the known elements in a meaningful way.
Mendeleev's insights came to a head as he worked on his book, "Principles of Chemistry," published in 1868. It was during this time that he began to consider the properties of elements in light of their atomic masses. Mendeleev famously wrote each element's known properties on a card and began organizing them. Through intense deliberation and some serendipitous inspiration, he arranged these elements in a table where patterns became apparent—elements with similar properties occurred at regular intervals, hinting at a deeper organizational principle.
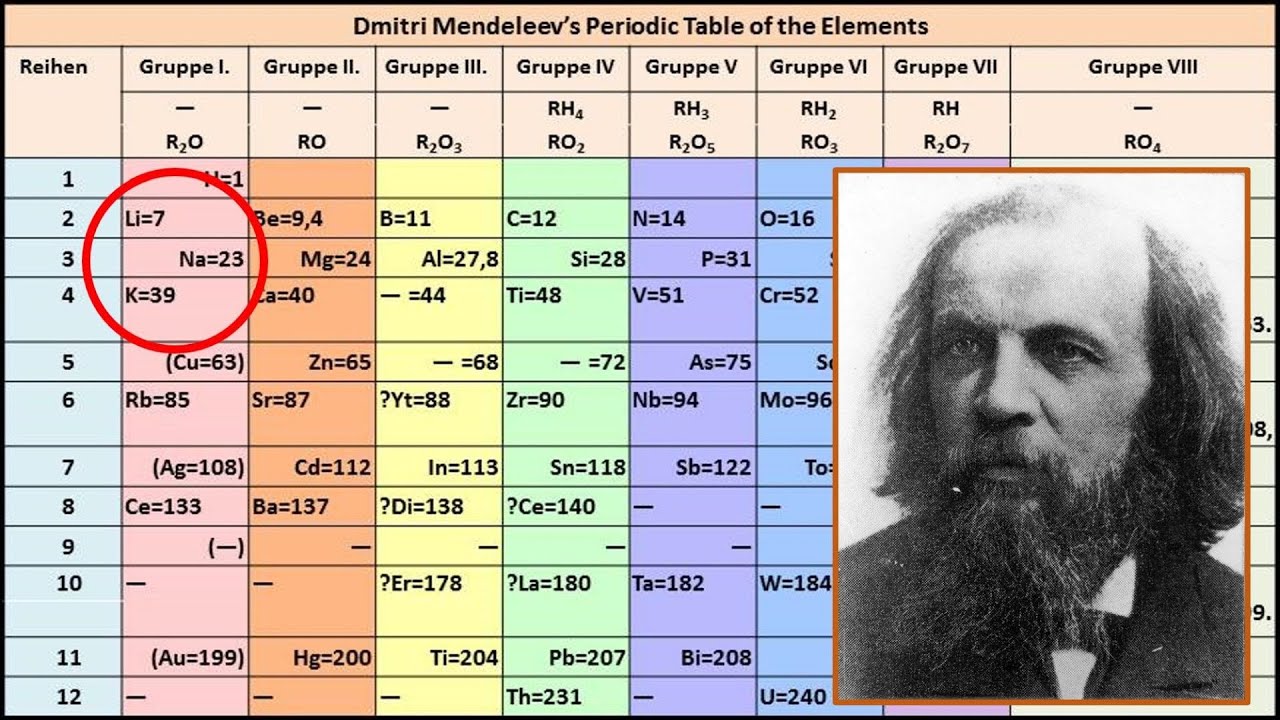
The First Periodic Table
In 1869, Mendeleev presented what became known as the first periodic table to the Russian Chemical Society. Unlike others before him who had attempted to organize elements, Mendeleev's breakthrough was in his recognition of periodicity and his courageous predictions about elements that had yet to be discovered. He left gaps in his table, suggesting that there were undiscovered elements that would fill these spaces, and even predicted their properties with remarkable accuracy.
For instance, he predicted the properties of germanium, gallium, and scandium—elements which were discovered years after his table was published and astonishingly fit into his predicted characteristics. This bold approach and these successful predictions validated his periodic system and laid a foundational stone for modern chemistry.
Legacy and Impact
Mendeleev’s periodic table was not initially accepted without debate. Critics questioned the validity of leaving gaps for unknown elements and the displacement of elements to prioritize properties over strict sequentiality of atomic weights. However, as the new elements were discovered and seamlessly aligned with his table, Mendeleev’s reputation soared.
The periodic table's significance extends far beyond the scientific community, influencing education, research, and industry. It serves as a roadmap of the elements, highlighting the periodic trends and giving clarity to the complex nature of chemical properties. Mendeleev’s work allowed scientists to predict and explore the behavior of elements under various conditions, thus contributing to advances in technology and materials.
Mendeleev was not just the architect of the periodic table but a prolific researcher and thinker. His contributions spanned various fields, including the study of solutions, the nature of gases, and the properties of chemical compounds. Mendeleev even ventured into non-scientific domains, exploring fields such as economics and meteorology.
The next stage of Mendeleev's journey brings us deeper into his life’s work and further developments that continued to shape the scientific narrative.
Scientific Methodology and Philosophy
Mendeleev’s success with the periodic table was not merely a product of his keen observational skills but also of his methodical approach to scientific inquiry. He exemplified the scientific method, meticulously gathering and analyzing data related to chemical properties and atomic weights. In organizing his table, Mendeleev emphasized the importance of valuing empirical evidence over theoretical models that lacked substantial support. This pragmatic approach set him apart from many contemporaries and fostered advancements not just in chemistry but also across scientific methodologies.
His commitment to empirical science is evident in his willingness to revise and adapt his models as new data became available. This adaptability was crucial, particularly in light of his predictions about undiscovered elements. By leaving gaps in his periodic table and hypothesizing about elements yet to be found, he demonstrated a profound understanding that science is an evolving discipline, open to refinement and revision.
The Mendeleev–Clark Discoveries
An often-overlooked aspect of Mendeleev's life is his collaboration with fellow scientists. One such partnership was with British chemist Alexander William Clarke, with whom he shared notes and findings. Together, they delved into atomic weights and spectral analysis, which, while occasionally contentious, ultimately proved invaluable to the advancement of Mendeleev's periodic system.
Both chemists realized that discrepancies in atomic weight measurements could lead to a deeper understanding of element properties—a notion that complemented Mendeleev's predictive abilities. This collaborative spirit exemplifies the network of ideas and cross-border cooperation that significantly enriched the scientific landscape of the era.
Contributions Beyond the Periodic Table
Though Mendeleev is most celebrated for his pivotal work on the periodic table, his scientific endeavors extended far beyond this singular achievement. He conducted extensive research in various domains, contributing substantially to the study of gases, solutions, and the expansion of the chemical industry—fields that were crucial to Russia's modernization during the industrial era.
For instance, Mendeleev’s work on the Ideal Gas Law, often overshadowed by his periodic table, provided critical insights into the behavior of gases under different conditions. While the law—PV = nRT—was earlier established in its specific form by other scientists, Mendeleev’s contributions to understanding and teaching this principle were influential in widespread adoption and application.
In the realm of applied chemistry, Mendeleev also made significant strides in the Russian oil industry. He recognized the immense potential of oil, advocating for its extraction and refining, and contributed to the development of techniques still foundational in modern petrochemical practices. His foresight in oil’s future utility speaks to his broader vision of integrating scientific knowledge into societal progress.
Educational Advocacy and Public Service
Mendeleev’s legacy extended into the realms of education and public service, where he fervently advocated for scientific learning and progressive pedagogy. A passionate educator, he sought to make chemistry accessible and engaging to students. He emphasized the importance of integrating current scientific discoveries into curriculum practices, urging reform in educational systems to accommodate the rapid proliferation of knowledge.
His service extended beyond the classroom. Mendeleev frequently advised the Russian government on matters of science and industry, recognizing the pivotal role of scientific advancement in national development. He served as a consultant on various governmental bodies focusing on metrology and standards, contributing to the establishment of stringent measurement systems vital to trade and industry—areas that were precursors to what we now know as national standardization organizations.
Mendeleev’s public service ethos was rooted in his belief that science should not be isolated to academia but applied to improve societal welfare. This philosophy underscored his advocacy for investment in scientific research and education to address Russia's significant economic and technological challenges in the latter half of the 19th century.
Recognition and Controversy
Despite his monumental contributions, Mendeleev's career was not free from controversy. Notably, he was repeatedly overlooked for the Nobel Prize in Chemistry, in part due to scientific politics and differences with other prominent chemists. Some suggested that his straightforward character and open criticism of established theories led to clashes within the scientific community, impacting his recognition on the Nobel stage.
Regardless, Mendeleev received numerous other accolades throughout his life, including membership in scientific societies across Europe and recognition from prestigious academies. His contributions were further honored by his legacy in the periodic table, forever marked with the element 101, Mendelevium, named in his honor posthumously in 1955.
As we continue to unfold Mendeleev’s story, we will explore the lasting implications of his work and how his insights paved the way for future scientific discoveries.
Mendeleev’s Influence on Modern Science
The reverberations of Dmitri Mendeleev’s work on the periodic table continue to be felt in modern science. His table laid the groundwork for the development of quantum mechanics and atomic theory in the early 20th century—fields that have vastly expanded our understanding of material science and chemistry. The predictability in element behavior that Mendeleev highlighted has enabled scientists to explore complex compounds and novel materials, fueling innovations across industries including electronics, pharmaceuticals, and nanotechnology.
Advances in atomic theory, such as the discovery of isotopes and the subsequent modification of the periodic table to reflect atomic number instead of atomic weight, reflect the dynamic nature of Mendeleev’s creation. These developments underscore his foresight in championing periodic trends, which remain pivotal in elemental science today.
The Periodic Table in Education
One of Mendeleev’s lasting contributions is the incorporation of the periodic table into scientific education. It serves as a fundamental tool for teaching chemistry worldwide, illustrating key concepts like electron configurations and chemical reactivity. The table’s logical organization aids in hypothesizing the behavior of elements, an essential skill for aspiring scientists.
The periodic table has also inspired educational methodologies aimed at critical thinking. By understanding the relationships and trends inherent to the table, students learn to extrapolate information, predict outcomes, and solve complex chemical problems—skills that are transferable to a variety of scientific disciplines.
Societal Impact and Future Relevant Insights
Beyond the confines of scientific discovery, Mendeleev's work also holds significant societal implications. In an age where new materials and elements are constantly being synthesized, the periodic table helps guide ethically informed science. By illustrating how elements interact under different conditions, it allows scientists to anticipate the environmental impacts of novel compounds and aids policymakers in establishing safety regulations and standards.
Looking towards the future, the periodic table continues to evolve. With researchers probing the limits of high atomic number elements and the quest for undiscovered superheavy elements, Mendeleev’s vision encourages an ever-expanding horizon of inquiry. The flexibility and adaptability first championed by Mendeleev remain at the forefront as we push the boundaries of scientific understanding.
Personal Life and Legacy
While Mendeleev's professional achievements were numerous, his personal life also reflects the complexities of his era. Mendeleev was married twice and had a total of eight children, balancing his personal endeavors with his scientific pursuits. His life was marked by a strong sense of duty to his family, his work, and his country, underpinning the multifaceted nature of his legacy.
His legacy lives on not just through his monumental scientific contributions but also through the lives and careers of his many students and collaborators who carried forward his passion for chemistry. His insistence on rigorous empirical research and education reforms helped spawn generations of scientists who contributed to the flourishing of chemistry as a global discipline.
Moreover, Mendeleev's life story exemplifies the essential human capacity for innovation and determination in the face of challenges. From overcoming personal hardships in a distant Siberian town to elevating Russian science on the international stage, Mendeleev’s remarkable journey is an enduring testament to the power of curiosity and relentless pursuit of knowledge.
Conclusion: A Timeless Influence
As the architect of the periodic table, Dmitri Mendeleev transformed the once fragmented field of chemistry into a cohesive scientific domain. His visionary framework not only enabled the discovery and integration of new elements but also facilitated advancements in manifold scientific disciplines. Today, the periodic table stands not merely as a symbol of chemical order but as a tribute to Mendeleev's enduring legacy.
Mendeleev's brilliance, driven by an unerring belief in the power of scientific inquiry, underscores the continuing journey of discovery that defines the scientific endeavor. As we explore the complexities of the atomic world, the impact of Mendeleev’s work remains a guiding beacon, affirming his rightful place among the great minds who have shaped our understanding of the universe.






Comments