Sardinian Genetic Law: Evolution and Disease Resistance
The unique genetic landscape of Sardinia presents a fascinating case study of evolution in action. Often referred to as a genetic island, the population's relative isolation has led to distinctive biological traits and health outcomes. This article explores the evolutionary pressures and legal frameworks that have shaped the Sardinian gene pool, revealing insights into disease resistance and longevity.
The Sardinian Genetic Isolation Phenomenon
Sardinia's status as a Mediterranean island has profoundly influenced its population genetics. Centuries of relative isolation have resulted in a homogeneous gene pool, making it an invaluable resource for scientific research. This genetic bottleneck has amplified the presence of certain variants, creating a unique natural laboratory for human genetics.
Studies comparing Sardinian DNA with other European populations reveal significant differences. The island's genetic makeup holds keys to understanding complex diseases and potential treatments. This isolation is not just a historical footnote but a living, breathing example of human adaptation.
Key Factors Driving Genetic Isolation
Several historical and geographical factors contributed to the distinct Sardinian genetic profile. The island's mountainous terrain limited internal migration and external contact for many generations.
- Geographical barriers reducing gene flow from mainland populations
- Historical patterns of settlement and limited colonization
- Cultural and linguistic traditions favoring endogamy
- Relatively stable population size over centuries
Malaria as a Major Evolutionary Force
Throughout history, malaria was endemic in Sardinia, exerting immense selective pressure on the population. The deadly Plasmodium falciparum parasite shaped the genetic destiny of the island's inhabitants, favoring mutations that conferred resistance.
This evolutionary arms race between humans and pathogens left a permanent mark on the Sardinian genome. Researchers have identified specific genetic adaptations that provided survival advantages against malaria. These mutations, however, often came with a trade-off, increasing susceptibility to other conditions.
Genetic Adaptations to Malaria
The most well-documented adaptation involves genes related to red blood cell structure and function. Mutations that slightly altered blood cells made it more difficult for the malaria parasite to thrive.
Genetic studies show that certain Sardinian variants, like those affecting Glucose-6-phosphate dehydrogenase (G6PD), provided significant protection against severe malaria. This enzyme deficiency disrupts the parasite's life cycle within red blood cells.
Other genetic factors influencing immune response also underwent selection. The evolutionary trade-off between malaria resistance and autoimmune risk is a central theme in Sardinian genetic research.
The High Prevalence of Autoimmune Diseases
The same genetic variations that protected Sardinians from malaria now contribute to one of the world's highest rates of autoimmune diseases. Conditions like multiple sclerosis, type 1 diabetes, and autoimmune thyroiditis are significantly more common on the island.
This phenomenon illustrates the concept of balancing selection, where a genetic variant is beneficial in one context but detrimental in another. The eradication of malaria in the mid-20th century removed the protective benefit of these genes, leaving only the increased autoimmune risk.
Specific Autoimmune Conditions in Sardinia
Research has quantified the increased prevalence of several autoimmune disorders. Type 1 diabetes incidence in Sardinia is among the highest globally, affecting approximately 40-50 per 100,000 children annually.
- Multiple sclerosis rates are double the European average
- High incidence of autoimmune thyroid disease
- Increased prevalence of celiac disease and other conditions
- Unique local autoimmune disorders rarely seen elsewhere
Sardinia's Blue Zone Longevity Paradox
Despite higher rates of certain diseases, Sardinia is famous as one of the world's Blue Zones, regions with exceptional longevity. This apparent contradiction highlights the complexity of health and aging, where genetics interact with lifestyle and environment.
The island, particularly the mountainous Ogliastra region, has an unusually high concentration of male centenarians. Researchers are investigating whether specific genetic factors contribute to this extended healthspan, potentially offsetting autoimmune risks.
Lifestyle Factors and Genetic Interactions
The traditional Sardinian lifestyle, characterized by physical activity, a plant-based diet, and strong social connections, likely modulates genetic predispositions. This gene-environment interaction offers crucial lessons for healthy aging worldwide.
Studies of Sardinian centenarians have identified potential longevity-associated genes that may protect against age-related decline. Understanding how these factors interact with autoimmune risk genes represents a major focus of current research.
The Genetic Architecture of Autoimmunity in Sardinia
The unique position of the Sardinian population has made it a focal point for genome-wide association studies. Researchers have identified multiple genetic loci that contribute significantly to the high prevalence of autoimmune disorders. These findings are not just academically interesting; they hold real-world implications for personalized medicine approaches.
One of the most studied regions is the HLA complex on chromosome 6. Certain HLA alleles, which are crucial for immune function, are present at much higher frequencies in Sardinians compared to other European populations. This genetic predisposition is a double-edged sword, offering historical advantages while creating modern health challenges.
Key Genetic Variants Identified
Several specific genetic markers have been strongly associated with Sardinian autoimmune diseases. The HLA-DRB1*03:01 and HLA-DRB1*04:05 alleles, for instance, show a powerful link to type 1 diabetes susceptibility.
- HLA-DQ2/DQ8 variants for celiac disease predisposition
- IRF5 and STAT4 genes linked to systemic lupus erythematosus risk
- PTPN22 gene variants associated with multiple autoimmune conditions
- Unique Sardinian-specific mutations in immune regulation pathways
The Thrifty Genotype Hypothesis in Sardinia
The thrifty genotype hypothesis suggests that genes which were once advantageous for survival in feast-or-famine conditions can become detrimental in modern environments. In Sardinia, this concept extends to immune function, where genes that provided survival advantages against infectious diseases now contribute to autoimmune conditions.
This evolutionary perspective helps explain why certain genetic variants persist at high frequencies. The protection these genes offered against pathogens like malaria was so significant that any negative effects were outweighed in historical contexts. Modern hygiene and medicine have removed these pressures, revealing the evolutionary trade-offs.
Research indicates that nearly 25% of the Sardinian population carries genetic variants that significantly increase autoimmune disease risk. This high frequency is a direct result of historical evolutionary pressures.
Metabolic and Immune Connections
The relationship between metabolism and immunity is particularly evident in Sardinian genetics. Genes involved in energy storage and utilization often have pleiotropic effects on immune function. This connection may explain comorbidities between metabolic and autoimmune disorders observed in the population.
Studies of Sardinian families have revealed how these genetic networks interact. The same pathways that regulated insulin sensitivity for survival during periods of food scarcity now influence immune cell function in ways that can lead to autoimmunity.
Environmental Triggers and Gene Expression
While genetics provide predisposition, environmental factors play a crucial role in determining whether autoimmune diseases manifest. The Sardinian environment has undergone significant changes in recent decades, potentially explaining the rising incidence of these conditions.
The hygiene hypothesis proposes that reduced exposure to microorganisms in childhood can lead to improperly regulated immune systems. As Sardinia has modernized, changes in sanitation, diet, and infectious disease exposure have likely interacted with genetic predispositions.
Dietary Changes and Microbiome Impacts
The traditional Sardinian diet, rich in whole grains, legumes, and vegetables, has shifted toward more processed foods and animal products. This dietary transition has profound effects on the gut microbiome, which plays a critical role in immune system education and regulation.
- Decreased consumption of fermented foods rich in beneficial bacteria
- Increased use of antibiotics and preservatives affecting microbial diversity
- Changes in fiber intake impacting gut barrier function
- Potential loss of protective microorganisms from traditional food preparation
Sardinian Genetic Research and Global Implications
The concentrated nature of genetic variants in Sardinia makes it an ideal natural laboratory for studying autoimmune diseases. Findings from Sardinian research have contributed significantly to our understanding of these conditions worldwide.
Several pharmaceutical developments have been informed by Sardinian genetic studies. By understanding the specific mechanisms through which these genetic variants contribute to disease, researchers can develop more targeted and effective treatments.
Contributions to Precision Medicine
Sardinian research has helped identify biomarkers for disease risk stratification and early detection. This knowledge enables more personalized approaches to prevention and treatment, potentially benefiting populations beyond Sardinia.
The island's genetic homogeneity reduces background noise in studies, making it easier to detect significant associations. This advantage has accelerated discovery in complex autoimmune conditions that involve multiple genetic and environmental factors.
Recent studies estimate that genetic insights from Sardinian populations could inform treatment strategies for up to 15% of autoimmune disease patients globally, highlighting the disproportionate impact of this research.
As genetic sequencing technologies advance, the Sardinian population continues to offer unique insights. The combination of detailed genealogical records and willingness to participate in research creates an unparalleled resource for understanding human health and disease.
Public Health Implications and Genetic Counseling
The unique genetic profile of Sardinia presents significant challenges and opportunities for public health planning and medical services. Healthcare providers on the island must balance the management of relatively common autoimmune conditions with the population's overall exceptional longevity. This requires specialized knowledge of local genetic predispositions and their clinical manifestations.
Genetic counseling services have become increasingly important for Sardinian families. Understanding inheritance patterns and risk assessment helps individuals make informed decisions about their health. The concentration of specific genetic variants allows for more accurate predictions than might be possible in more genetically diverse populations.
Developing Targeted Screening Programs
Based on the identified genetic risks, Sardinia has implemented population-specific screening initiatives. These programs aim for early detection of conditions like type 1 diabetes and celiac disease, allowing for timely intervention and management.
- Newborn screening for high-risk genetic markers
- Regular autoantibody testing for at-risk individuals
- Specialized monitoring for families with multiple affected members
- Community education about early symptoms and risk factors
Future Research Directions in Sardinian Genetics
The next frontier in Sardinian genetic research involves exploring the epigenetic modifications that influence gene expression. Scientists are investigating how environmental factors trigger autoimmune responses in genetically predisposed individuals. This research could reveal new pathways for prevention and treatment.
Longitudinal studies tracking generational changes in gene expression and disease incidence are underway. As Sardinia continues to modernize, researchers can observe how genetic predispositions interact with changing lifestyles. These studies provide real-time insights into gene-environment interactions.
Current research projects involve over 10,000 Sardinian participants in multi-generational studies, providing unprecedented data on genetic and environmental interactions over time.
Pharmacogenomics and Personalized Treatments
The unique genetic makeup of Sardinians has implications for drug development and prescription. Research is focusing on how common genetic variants affect medication metabolism and efficacy. This knowledge enables more personalized treatment approaches with fewer side effects.
Several pharmaceutical companies are collaborating with Sardinian research institutions to develop targeted therapies for autoimmune conditions. The homogeneous genetic background provides an ideal testing ground for medications that might work specifically for certain genetic profiles.
Ethical Considerations in Genetic Research
The concentrated nature of Sardinian genetics raises important ethical questions about privacy, consent, and the potential for genetic discrimination. Researchers and ethicists are working together to establish guidelines that protect participants while advancing scientific knowledge.
Issues of informed consent are particularly important in small, closely-knit communities. Participants must understand how their genetic information might be used and shared. Robust protocols ensure that research benefits the community while respecting individual rights.
Balancing Scientific Progress and Cultural Sensitivity
Genetic research in Sardinia requires careful attention to cultural traditions and community values. Researchers work closely with local leaders to ensure that studies are conducted respectfully and that findings are communicated appropriately back to the community.
- Establishing community advisory boards for research oversight
- Developing culturally appropriate consent processes
- Ensuring equitable benefit sharing from research findings
- Protecting against genetic stigmatization of the population
Global Lessons from Sardinian Genetics
The Sardinian genetic story offers valuable insights for global health beyond the island's shores. The principles of evolutionary trade-offs observed in Sardinia likely apply to other populations with distinct genetic histories. Understanding these patterns can inform public health strategies worldwide.
The concept of balancing selection demonstrated in Sardinia helps explain disease patterns in other isolated or founder populations. This knowledge allows healthcare systems to anticipate and prepare for population-specific health challenges.
Applications to Migrant Health
As Sardinians migrate to other regions, their genetic predispositions travel with them. Healthcare providers in destination countries need awareness of these population-specific health risks. This understanding becomes increasingly important in our globalized world with significant population mobility.
Similarly, the Sardinian model of genetic research integration with clinical care provides a template for other populations. The successful collaboration between researchers, clinicians, and the community offers lessons in translational medicine.
Conclusion: The Sardinian Genetic Legacy
The Sardinian population represents a unique natural experiment in human genetics and evolution. The island's history of isolation, combined with specific environmental pressures, has created a genetic profile that offers profound insights into human health and disease. The evolutionary trade-offs observed provide a powerful framework for understanding complex disease patterns.
Key takeaways from Sardinian genetic research highlight the importance of population-specific medicine. The high prevalence of autoimmune diseases alongside exceptional longevity demonstrates the complexity of genetic influences on health. These apparent contradictions underscore the need for nuanced approaches to healthcare and research.
Final Implications for Science and Society
The Sardinian story reminds us that our genetic heritage is a double-edged sword. Traits that provided survival advantages in one context may create vulnerabilities in another. This understanding encourages humility in how we approach both genetic research and clinical practice.
As genetic technologies advance, the lessons from Sardinia will become increasingly relevant. The island's experience with precision medicine implementation, ethical considerations, and community engagement provides a valuable model for the future of healthcare. The Sardinian genetic legacy continues to shape our understanding of what it means to be human in a biological sense.
The ongoing research in Sardinia demonstrates that approximately 60% of the population's disease risk profile can be traced to specific genetic variants shaped by historical evolutionary pressures, offering unprecedented insight into human adaptation.
The future of Sardinian genetic research promises even deeper understanding of the intricate balance between our evolutionary past and modern health challenges. As science continues to unravel these complex relationships, the island's genetic story will undoubtedly continue to provide crucial insights for generations to come, benefiting not only Sardinians but people worldwide facing similar genetic complexities.
Understanding HLA: The Immune System's Genetic Blueprint
What is Human Leukocyte Antigen (HLA)?
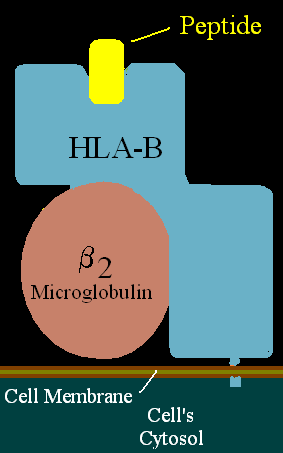
The Human Leukocyte Antigen (HLA) system is a critical component of the human immune system. Located on chromosome 6, these genes encode cell-surface proteins that play a pivotal role in regulating immune responses. By presenting peptide antigens to T cells, HLA molecules help the body distinguish between self and non-self cells, a fundamental process in immune defense.
The Structure and Function of HLA
Class I and Class II HLA Molecules
HLA molecules are categorized into two main classes: Class I (HLA-A, B, C) and Class II (HLA-DR, DQ, DP). Class I molecules are present on nearly all nucleated cells and are responsible for displaying intracellular peptides, such as those derived from viruses, to CD8+ cytotoxic T cells. This interaction is crucial for the elimination of infected or malignant cells.
Class II molecules, on the other hand, are found on antigen-presenting cells and present extracellular antigens to CD4+ helper T cells. This process is essential for initiating and coordinating immune responses against pathogens.
Class III Genes
In addition to Class I and II, HLA also includes Class III genes, which encode proteins involved in inflammation, such as complement components and tumor necrosis factor-alpha (TNF-alpha). These proteins play a significant role in the body's inflammatory responses and overall immune regulation.
The Role of HLA in Immune Regulation
Distinguishing Self from Non-Self
The primary function of HLA is to distinguish between self and non-self cells. This is achieved through the presentation of peptide antigens to T cells. In a healthy state, HLA molecules suppress the presentation of self-antigens, preventing autoimmune responses. Disruptions in this process can lead to autoimmunity, where the immune system mistakenly attacks the body's own cells.
Influence on Disease Susceptibility
Variations in HLA genes can influence an individual's susceptibility to certain diseases. For example, specific HLA alleles have been linked to an increased risk of developing autoimmune diseases such as multiple sclerosis (MS) and severe infections. Understanding these genetic variations is crucial for developing personalized treatment strategies.
The Importance of HLA in Transplantation
Matching Donors and Recipients
HLA typing is essential for matching donors and recipients in organ and stem cell transplants. A close match between the donor and recipient HLA types minimizes the risk of transplant rejection. Incompatible HLA molecules can trigger host T-cell or antibody responses, leading to graft rejection.
Transplant Success and HLA Matching
The success of a transplant is significantly influenced by the degree of HLA matching. A 6/6 HLA match is considered ideal for unrelated donors. Mismatches can increase the risk of rejection by 20-50%, highlighting the importance of precise HLA typing in transplant procedures.
Recent Advances in HLA Research
Precision Medicine and Immunotherapy
Recent trends in HLA research emphasize the role of precision medicine. Advances in HLA typing are enhancing the effectiveness of immunotherapies, such as CAR-T cells and cancer vaccines. By targeting allele-specific peptide presentation, these therapies can be tailored to individual patients, improving treatment outcomes.
Computational Models for HLA-Peptide Binding
Improving computational models for HLA-peptide binding is another area of active research. These models support the development of personalized vaccines by predicting how different HLA alleles will interact with specific peptides. This approach holds great promise for the future of personalized medicine.
Conclusion
The Human Leukocyte Antigen (HLA) system is a cornerstone of the human immune system, playing a vital role in distinguishing self from non-self cells and regulating immune responses. Its significance in transplantation, disease susceptibility, and precision medicine underscores the importance of ongoing research and advancements in HLA typing and computational modeling.
The Genetic Diversity of HLA: A Double-Edged Sword
Extreme Polymorphism and Its Implications
The HLA system is renowned for its extreme polymorphism, with over 20,000 alleles identified across various loci. This genetic diversity is a double-edged sword: it enhances the body's ability to recognize a wide range of pathogens but also complicates transplantation processes. Each individual inherits one set of HLA genes from each parent, resulting in a unique combination that influences immune responses.
Heterozygosity and Pathogen Recognition
Most individuals are heterozygous at HLA loci, meaning they have different alleles for each gene. This heterozygosity is advantageous as it broadens the spectrum of peptides that can be presented to T cells, thereby enhancing pathogen recognition. However, this diversity also means that finding a perfect match for organ transplants can be challenging.
HLA and Autoimmune Diseases: The Connection
HLA Alleles and Disease Susceptibility
Certain HLA alleles have been strongly associated with an increased risk of developing autoimmune diseases. For instance, specific variants of HLA-DRB1 are linked to conditions such as rheumatoid arthritis and multiple sclerosis. These associations highlight the critical role of HLA in maintaining immune tolerance and preventing autoimmune responses.
Mechanisms of Autoimmunity
In autoimmunity, the immune system fails to distinguish between self and non-self antigens, leading to the destruction of healthy tissues. HLA molecules play a pivotal role in this process by presenting self-antigens to T cells. When this presentation goes awry, it can trigger an autoimmune response. Understanding these mechanisms is crucial for developing targeted therapies.
HLA in Cancer Immunity and Immunotherapy
Tumor Surveillance and HLA
HLA molecules are integral to the body's ability to surveil and eliminate cancerous cells. They present tumor-specific antigens to T cells, which can then mount an immune response against the tumor. However, cancer cells often evolve mechanisms to evade this surveillance, such as downregulating HLA expression or altering the peptides presented.
Advances in Cancer Immunotherapy
Recent advances in cancer immunotherapy have leveraged the HLA system to enhance the body's natural defenses against tumors. Techniques such as CAR-T cell therapy and cancer vaccines are designed to target specific HLA-peptide complexes, thereby improving the precision and effectiveness of these treatments. These innovations hold great promise for the future of cancer treatment.
The Role of HLA in Pregnancy and Alloimmunization
Maternal-Fetal HLA Interactions
During pregnancy, the maternal immune system must tolerate the presence of fetal cells that express paternal HLA molecules. This tolerance is crucial for a successful pregnancy. However, in some cases, the maternal immune system may develop antibodies against these foreign HLA molecules, leading to complications such as alloimmunization.
Alloimmunization and Its Consequences
Alloimmunization can occur not only during pregnancy but also as a result of blood transfusions or organ transplants. When the immune system is exposed to foreign HLA molecules, it may produce antibodies that can attack these molecules, leading to transplant rejection or other complications. Understanding and managing alloimmunization is essential for improving the outcomes of these medical procedures.
Computational Models and HLA-Peptide Binding
Predicting HLA-Peptide Interactions
Computational models are increasingly being used to predict how different HLA alleles will interact with specific peptides. These models are based on extensive databases of HLA-peptide binding data and use machine learning algorithms to make accurate predictions. This approach is particularly useful for developing personalized vaccines and immunotherapies.
Applications in Personalized Medicine
The use of computational models in HLA research is revolutionizing the field of personalized medicine. By accurately predicting HLA-peptide interactions, researchers can design vaccines and therapies that are tailored to an individual's unique HLA profile. This personalized approach has the potential to significantly improve the efficacy and safety of medical treatments.
Challenges and Future Directions in HLA Research
Overcoming Transplant Rejection
One of the major challenges in HLA research is overcoming transplant rejection. Despite advances in HLA typing and matching, finding a perfect match for organ transplants remains difficult. Future research aims to develop new strategies for inducing immune tolerance and reducing the risk of rejection, thereby improving transplant outcomes.
Enhancing Immunotherapy Efficacy
Another key area of focus is enhancing the efficacy of immunotherapies. While current immunotherapies have shown promise, they are not effective for all patients. Future research aims to identify new targets and develop more precise therapies that can overcome the limitations of current treatments.
Conclusion
The Human Leukocyte Antigen (HLA) system is a complex and dynamic component of the human immune system. Its role in distinguishing self from non-self, regulating immune responses, and influencing disease susceptibility underscores its importance in health and medicine. Ongoing research and advancements in HLA typing, computational modeling, and immunotherapy hold great promise for the future of personalized medicine and transplant success.
HLA Testing: Methods and Clinical Applications
Traditional HLA Typing Techniques
Historically, HLA typing relied on serological methods, where antibodies were used to identify specific HLA antigens on cells. While effective, these techniques had limitations in resolution and specificity. Modern molecular methods, such as PCR-based sequencing, have revolutionized HLA typing by providing higher resolution and accuracy.
Next-Generation Sequencing (NGS) in HLA Typing
The advent of Next-Generation Sequencing (NGS) has significantly advanced HLA typing capabilities. NGS allows for high-throughput sequencing of HLA genes, enabling the identification of novel alleles and providing a more comprehensive understanding of an individual's HLA profile. This technology is particularly valuable in transplant matching and disease association studies.
The Impact of HLA on Drug Hypersensitivity
HLA-Associated Adverse Drug Reactions
Certain HLA alleles are strongly associated with an increased risk of adverse drug reactions. For example, the HLA-B*57:01 allele is linked to hypersensitivity reactions to the HIV drug abacavir. Identifying these associations is crucial for predicting and preventing adverse drug reactions, thereby improving patient safety.
Pharmacogenomics and HLA
The field of pharmacogenomics explores how genetic variations, including those in HLA genes, influence drug responses. By integrating HLA typing into pharmacogenomic testing, healthcare providers can tailor drug therapies to individual patients, minimizing the risk of adverse reactions and optimizing treatment efficacy.
HLA and Infectious Disease Susceptibility
HLA Variants and Pathogen Resistance
Specific HLA variants have been shown to confer resistance or susceptibility to certain infectious diseases. For instance, the HLA-B*53 allele is associated with protection against severe malaria. Understanding these genetic associations can provide valuable insights into the mechanisms of infectious disease resistance and inform the development of targeted therapies.
HLA in Viral Infections
HLA molecules play a critical role in the immune response to viral infections. They present viral peptides to T cells, initiating an immune response. However, some viruses have evolved mechanisms to evade HLA-mediated immunity, such as downregulating HLA expression or producing proteins that interfere with antigen presentation. Research in this area is essential for developing effective antiviral therapies.
Ethical Considerations in HLA Research and Applications
Privacy and Genetic Data
The use of HLA typing and genetic data raises important ethical considerations, particularly regarding privacy and data security. As HLA typing becomes more widespread, it is crucial to establish robust protocols for protecting individuals' genetic information and ensuring that it is used responsibly and ethically.
Equity in Access to HLA-Based Therapies
Ensuring equitable access to HLA-based therapies is another critical ethical issue. Advances in personalized medicine and immunotherapy should be accessible to all individuals, regardless of socioeconomic status or geographic location. Addressing disparities in access to these technologies is essential for promoting health equity.
The Future of HLA Research: Innovations and Breakthroughs
CRISPR and HLA Gene Editing
The emergence of CRISPR-Cas9 gene editing technology holds immense potential for HLA research. By precisely modifying HLA genes, researchers can explore new avenues for treating autoimmune diseases, improving transplant outcomes, and enhancing cancer immunotherapies. This technology could revolutionize the field of HLA-based medicine.
Artificial Intelligence in HLA Research
Artificial intelligence (AI) is increasingly being integrated into HLA research to analyze vast datasets and predict HLA-peptide interactions. AI algorithms can identify patterns and correlations that may not be apparent through traditional methods, accelerating the discovery of new therapeutic targets and improving the precision of personalized medicine.
Conclusion: The Pivotal Role of HLA in Health and Medicine
The Human Leukocyte Antigen (HLA) system is a cornerstone of the human immune system, playing a vital role in distinguishing self from non-self, regulating immune responses, and influencing disease susceptibility. From its critical function in transplantation to its impact on autoimmune diseases, cancer immunity, and infectious disease resistance, HLA is integral to numerous aspects of health and medicine.
Advances in HLA typing techniques, such as Next-Generation Sequencing, have significantly enhanced our ability to understand and utilize HLA information. These advancements, combined with innovations in gene editing and artificial intelligence, are paving the way for groundbreaking therapies and personalized medical approaches.
As we continue to unravel the complexities of the HLA system, it is essential to address ethical considerations and ensure equitable access to HLA-based technologies. By doing so, we can harness the full potential of HLA research to improve health outcomes and transform the landscape of modern medicine.
In conclusion, the HLA system stands as a testament to the intricate and dynamic nature of the human immune system. Its profound impact on health and disease underscores the importance of ongoing research and innovation in this field. As we look to the future, the possibilities for HLA-based therapies and personalized medicine are boundless, offering hope for improved treatments and enhanced quality of life for individuals worldwide.